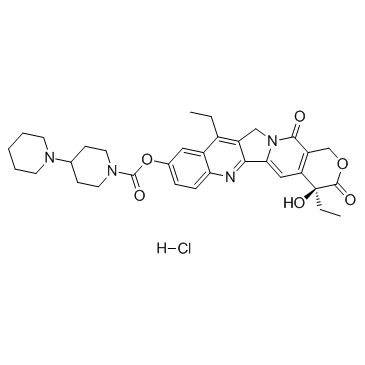
Irinotecan HCI Trihydrate
Modify Date: 2025-08-23 23:47:39
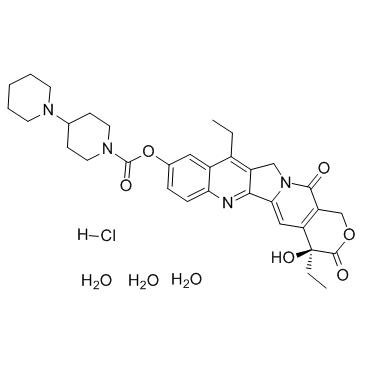
Irinotecan HCI Trihydrate structure
|
Common Name | Irinotecan HCI Trihydrate | ||
|---|---|---|---|---|
| CAS Number | 136572-09-3 | Molecular Weight | 677.185 | |
| Density | N/A | Boiling Point | 873.4ºC at 760 mmHg | |
| Molecular Formula | C33H45ClN4O9 | Melting Point | 250-256ºC (dec.) | |
| MSDS | N/A | Flash Point | 482ºC | |
Use of Irinotecan HCI TrihydrateIrinotecan hydrochloride trihydrate is a water soluble topoisomerase I inhibitor with antitumor activity. |
| Name | Irinotecan Hydrochloride Trihydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Irinotecan hydrochloride trihydrate is a water soluble topoisomerase I inhibitor with antitumor activity. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I |
| In Vitro | Irinotecan hydrochloride trihydrate is a topoisomerase I inhibitor. Irinotecan inhibits the growth of LoVo and HT-29 cells, with IC50s of 15.8 ± 5.1 and 5.17 ± 1.4 μM, respectively, and induces similar amounts of cleavable complexes in both in LoVo and HT-29 cells[2]. Irinotecan suppresses the proliferation of human umbilical vein endothelial cells (HUVEC), with an IC50 of 1.3 μM[3]. |
| In Vivo | Irinotecan (CPT-11, 5 mg/kg) significantly inhibits the growth of tumors by intratumoral injection daily for 5 days, on two consecutive weeks in rats, and such effects also occur via continuous intraperitoneal infusion by osmotic minipump into mice. However, Irinotecan (10 mg/kg) shows no effect on the growth of tumor by i.p[1]. Irinotecan (CPT-11, 100-300 mg/kg, i.p.) apparently suppresses tumor growth of HT-29 xenografts in athymic female mice by day 21. The two groups of Irinotecan (125 mg/kg) plus TSP-1 (10 mg/kg per day) or Irinotecan (150 mg/kg) in combination TSP-1 (20 mg/kg per day) are nearly equally effective and inhibit tumor growth 84% and 89%, respectively, and both are more effective than Irinotecan alone at doses of 250 and 300 mg/kg[3]. |
| Cell Assay | Exponentially growing cells are seeded in 20 cm2 dishes with an optimal cell number for each cell line (20,000 for LoVo cells, 100,000 for HT-29 cells). They are treated 2 days later with increasing concentrations of irinotecan or SN-38 for one cell doubling time (24 h for LoVo cells, 40 h for HT-29 cells). After washing with 0.15 M NaCl, the cells are further grown for two doubling times in normal medium, detached from the support with trypsin-EDTA and counted in a hemocytometer. The IC50 values are then estimated as the drug concentrations responsible for 50% growth inhibition as compared with cells incubated without drug[2]. |
| Animal Admin | Irinotecan has been administered by intratumoral injection at 0.1 cc volume of the appropriate solution, for a doses of 5 mg/kg daily for 5 days, on two consecutive weeks, followed by a 7-days rest period, referred to as one cycle of therapy. Rats receive three cycles over a period of 8 weeks. Control animals receive 0.1 cc of sterile 0.9% sodium chloride solution by intratumoral injection in the same rule of administration as that of animals of group II[1]. |
| References |
| Boiling Point | 873.4ºC at 760 mmHg |
|---|---|
| Melting Point | 250-256ºC (dec.) |
| Molecular Formula | C33H45ClN4O9 |
| Molecular Weight | 677.185 |
| Flash Point | 482ºC |
| Exact Mass | 676.287537 |
| PSA | 141.89000 |
| LogP | 4.57600 |
| InChIKey | KLEAIHJJLUAXIQ-JDRGBKBRSA-N |
| SMILES | CCc1c2c(nc3ccc(OC(=O)N4CCC(N5CCCCC5)CC4)cc13)-c1cc3c(c(=O)n1C2)COC(=O)C3(O)CC.Cl.O.O.O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| HS Code | 2934999090 |
|
~85% 
Irinotecan HCI ... CAS#:136572-09-3 |
| Literature: FERMION OY Patent: WO2006/84941 A2, 2006 ; Location in patent: Page/Page column 6 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| (+)-(4S)-4,11-Diethyl-4-hydroxy-9-<(piperidinopiperidino)-carbonyloxy>-1H-pyrano<3',4':6,7>indolizino<1,2-b>quinoline-3,14-(4H,12H)-dione hydrochloride trihydrate |
| [1,4'-Bipiperidine]-1'-carboxylic acid, (4S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl ester, hydrochloride, hydrate (1:1:3) |
| CPT-11 Trihydrate |
| Irinotecan hydrochloride trihydrate |
| MFCD01765731 |
| Irinotecan HCl Trihydrate |
| (4S)-4,11-Diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl 1,4'-bipiperidine-1'-carboxylate hydrochloride trihydrate |
| Irinotecan (hydrochloride trihydrate) |