abacavir sulfate
Modify Date: 2025-08-27 20:02:34
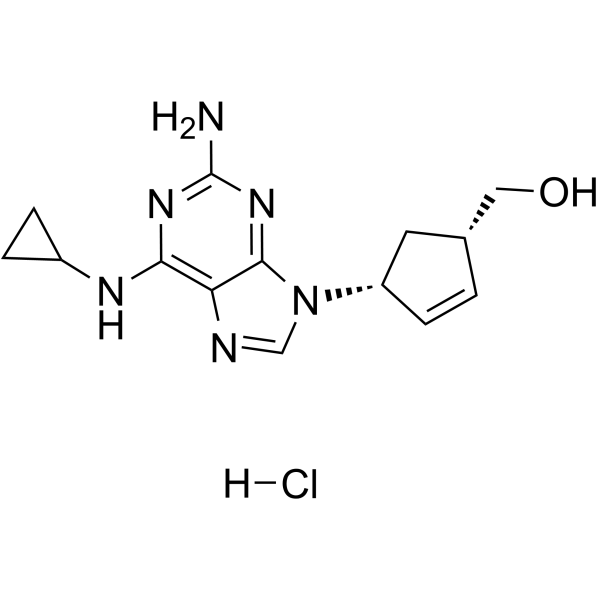
abacavir sulfate structure
|
Common Name | abacavir sulfate | ||
|---|---|---|---|---|
| CAS Number | 136777-48-5 | Molecular Weight | 670.74300 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C28H38N12O6S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of abacavir sulfateAbacavir hydrochloride is a competitive, orally active nucleoside reverse transcriptase inhibitor. Abacavir hydrochloride can inhibits the replication of HIV. Abacavir hydrochloride shows anticancer activity in prostate cancer cell lines. Abacavir hydrochloride can trespass the blood-brain-barrier and suppresses telomerase activity[1][2][3]. |
| Name | [(4S)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopenten-1-yl]methanol,sulfuric acid |
|---|
| Description | Abacavir hydrochloride is a competitive, orally active nucleoside reverse transcriptase inhibitor. Abacavir hydrochloride can inhibits the replication of HIV. Abacavir hydrochloride shows anticancer activity in prostate cancer cell lines. Abacavir hydrochloride can trespass the blood-brain-barrier and suppresses telomerase activity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Abacavir hydrochloride (15 and 150 μM, 0-120 h) inhibits cell growth, affects cell cycle progression, induces senescence and modulates LINE-1 mRNA expression in prostate cancer cell lines[1]. Abacavir hydrochloride (15 and 150 μM, 18 h) significantly reduces cell migration and inhibits cell invasion[1]. Abacavir hydrochloride induces fat apoptosis[4]. Cell Proliferation Assay[1] Cell Line: PC3, LNCaP and WI-38 Concentration: 15 μM and 150 μM Incubation Time: 0, 24, 48, 72 and 96 hours Result: Showed a dose-dependent growth inhibition on PC3 and LNCaP. Cell Cycle Analysis[1] Cell Line: PC3, LNCaP and WI-38 Concentration: 150 μM Incubation Time: 0, 18, 24, 48, 72, 96 and 120 hours Result: Caused a very high accumulation of cells in S phase in PC3 and LNCaP cells, and G2/M phase increment was observed in PC3 cells. Cell Migration Assay [1] Cell Line: PC3, LNCaP and WI-38 Concentration: 15 and 150 μM Incubation Time: 18 hours Result: Significantly reduced cell migration. Cell Invasion Assay[1] Cell Line: PC3, LNCaP and WI-38 Concentration: 15 and 150 μM Incubation Time: 18 hours Result: Significantly inhibited cell invision. |
| In Vivo | Abacavir hydrochloride (100 and 200 mg/kg, p.o.; 4 h) dose-dependently promotes thrombus formation[2]. Abacavir hydrochloride (50 mg/kg/d; i.p.; 14 d) with 0.1 mg/kg/d Decitabine (HY-A0004) enhances survival of high-risk medulloblastoma-bearing mice[3]. Animal Model: Male mice (9-weeks old, 22-30 g) - wild-type (WT) C57BL/6 or homozygous knockout (P2rx7 KO, B6.129P2-P2rx7tm1Gab/J)[2] Dosage: Route 1: 2.5, 5, and 7.5 μg/mL, 100 μL Route 2: 100 and 200 mg/kg Administration: Intrascrotal or oral administration for 4 h Result: Dose-dependently promoted thrombus formation. Animal Model: NSGTM mice, patient-derived xenograft (PDX) cells of non-WNT/non-SHH, Group 3 and of SHH/ TP53-mutated medulloblastoma[3] Dosage: 50 mg/kg/d with 0.1 mg/kg/d Decitabine Administration: Intraperitoneal injection, daily for 14 days Result: Inhibited tumor growth and enhanced mouse survival. |
| References |
| Molecular Formula | C28H38N12O6S |
|---|---|
| Molecular Weight | 670.74300 |
| Exact Mass | 670.27600 |
| PSA | 286.74000 |
| LogP | 3.92100 |
| InChIKey | QXNOPHSMRJNHHG-SCYNACPDSA-N |
| SMILES | Cl.Nc1nc(NC2CC2)c2ncn(C3C=CC(CO)C3)c2n1 |