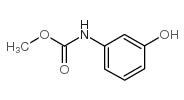
3-((Methoxycarbonyl)amino)phenyl m-tolylcarbamate

3-((Methoxycarbonyl)amino)phenyl m-tolylcarbamate structure
|
Common Name | 3-((Methoxycarbonyl)amino)phenyl m-tolylcarbamate | ||
|---|---|---|---|---|
| CAS Number | 13684-63-4 | Molecular Weight | 300.309 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 510.3±60.0 °C at 760 mmHg | |
| Molecular Formula | C16H16N2O4 | Melting Point | 140-144°C | |
| MSDS | Chinese USA | Flash Point | 262.4±32.9 °C | |
| Symbol |

GHS09 |
Signal Word | Warning | |
Use of 3-((Methoxycarbonyl)amino)phenyl m-tolylcarbamatePhenmedipham is a carbamate herbicide[1]. |
| Name | phenmedipham |
|---|---|
| Synonym | More Synonyms |
| Description | Phenmedipham is a carbamate herbicide[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 510.3±60.0 °C at 760 mmHg |
| Melting Point | 140-144°C |
| Molecular Formula | C16H16N2O4 |
| Molecular Weight | 300.309 |
| Flash Point | 262.4±32.9 °C |
| Exact Mass | 300.110992 |
| PSA | 76.66000 |
| LogP | 4.42 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.569 |
| Water Solubility | <0.1 g/100 mL at 21 ºC |
|
~99% 
3-((Methoxycarb... CAS#:13684-63-4 |
| Literature: Kemisk Vaerk Koge A/S Patent: US4748265 A1, 1988 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924299038 |
|---|---|
| Summary | 2924299038 2-chloro-n-(ethoxymethyl)-n-(2-ethyl-6-methylphenyl)acetamide。supervision conditions:s(import or export registration certificate for pesticides)。VAT:17.0%。tax rebate rate:9.0%。MFN tarrif:6.5%。general tariff:30.0% |
|
Mining biologically-active molecules for inhibitors of fatty acid amide hydrolase (FAAH): Identification of phenmedipham and amperozide as FAAH inhibitors
Bioorg. Med. Chem. Lett. 19 , 6793-6, (2009) The screening of known medicinal agents against new biological targets has been shown to be a valuable approach for revealing new pharmacology of marketed compounds. Recently, carbamate, urea and keto... |
|
|
The influence of lipophilicity and formulation on the distribution of pesticides in laboratory-scale sediment/water systems.
Pest Manag. Sci. 59(2) , 238-44, (2003) Pesticide reaching surface waters will be sorbed by sediment. This sorption process and the influence of pesticide formulation have been examined at 10 degrees C in small-scale systems having 2-cm dep... |
|
|
Purification and properties of an Arthrobacter oxydans P52 carbamate hydrolase specific for the herbicide phenmedipham and nucleotide sequence of the corresponding gene.
J. Bacteriol. 174(20) , 6600-7, (1992) Arthrobacter oxydans P52 isolated from soil samples was found to degrade the phenylcarbamate herbicides phenmedipham and desmedipham cometabolically by hydrolyzing their central carbamate linkages. Th... |
| 3-(methoxyformamido)phenyl (3-methylphenyl)carbamate |
| Phenmedipham |
| Methanol, 1-[3-[[(1E)-hydroxymethoxymethylene]amino]phenoxy]-1-[(3-methylphenyl)imino]-, (E)- |
| 3-((Methoxycarbonyl)amino)phenyl m-tolylcarbamate |
| Phenmedipham (3-Methoxycarbonylaminophenyl-N-3'-methylphenyl |
| 3-{(E)-[Hydroxy(methoxy)methylene]amino}phenyl hydrogen (3-methylphenyl)carbonimidate |
| EINECS 237-199-0 |
| MFCD00055419 |
| 3-[(methoxycarbonyl)amino]phenyl N-(3-methylphenyl)carbamate |
| 3-methoxycarbonylaminophenyl 3-methylcarbanilate |
| 3-((Methoxycarbonyl)amino)phenyl (3-methylphenyl)carbamate |
| 3-[(Methoxycarbonyl)amino]phenyl (3-methylphenyl)carbamate |
| 3-[(methoxycarbonyl)amino]phenyl (3-methylphenyl)carbamate (non-preferred name) |
| methyl 3-(3-methylcarbaniloyloxy)carbanilate |