Sulfathiazole sodium

Sulfathiazole sodium structure
|
Common Name | Sulfathiazole sodium | ||
|---|---|---|---|---|
| CAS Number | 144-74-1 | Molecular Weight | 277.298 | |
| Density | N/A | Boiling Point | 479.5ºC at 760mmHg | |
| Molecular Formula | C9H8N3NaO2S2 | Melting Point | 175ºC (form a); 202ºC (form b) | |
| MSDS | Chinese USA | Flash Point | 243.8ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Sulfathiazole sodiumSulfathiazole Sodium is an organosulfur compound that has been used as a short-acting sulfa drug.Target: AntibacterialSulfathiazole (20 μg/L) starts to be degraded between day 31 and day 38 in one of the two batch reactors containing different wastewater matrices. Sulfathiazole is degraded at a substantially faster rate than sulfamethoxazole or sulfamethazine in the nitrification process (S3) [1]. Recovery from spiked manure slurry samples is 64% for Sulfathiazole at pH 9. Sulfathiazole has acidity constant of pKa of 7.1and retention times (tR) of 7.8. S/N values for Sulfathiazole are above 100 at the 1 mg/kg level [2]. Sulfathiazole sorption to inorganic sorbents exhibits pronounced pH dependence consistent with sorbate speciation and sorbent charge properties. Sulfathiazole cations are most important for sorption to clay minerals, followed by neutral species [3]. |
| Name | Sulfathiazole sodium salt |
|---|---|
| Synonym | More Synonyms |
| Description | Sulfathiazole Sodium is an organosulfur compound that has been used as a short-acting sulfa drug.Target: AntibacterialSulfathiazole (20 μg/L) starts to be degraded between day 31 and day 38 in one of the two batch reactors containing different wastewater matrices. Sulfathiazole is degraded at a substantially faster rate than sulfamethoxazole or sulfamethazine in the nitrification process (S3) [1]. Recovery from spiked manure slurry samples is 64% for Sulfathiazole at pH 9. Sulfathiazole has acidity constant of pKa of 7.1and retention times (tR) of 7.8. S/N values for Sulfathiazole are above 100 at the 1 mg/kg level [2]. Sulfathiazole sorption to inorganic sorbents exhibits pronounced pH dependence consistent with sorbate speciation and sorbent charge properties. Sulfathiazole cations are most important for sorption to clay minerals, followed by neutral species [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 479.5ºC at 760mmHg |
|---|---|
| Melting Point | 175ºC (form a); 202ºC (form b) |
| Molecular Formula | C9H8N3NaO2S2 |
| Molecular Weight | 277.298 |
| Flash Point | 243.8ºC |
| Exact Mass | 276.995575 |
| PSA | 112.91000 |
| LogP | 3.04690 |
| Vapour Pressure | 2.35E-09mmHg at 25°C |
| InChIKey | GWIJGCIVKLITQK-UHFFFAOYSA-N |
| SMILES | Nc1ccc(S(=O)(=O)[N-]c2nccs2)cc1.[Na+] |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25-S23 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | WP2450000 |
| HS Code | 2935009090 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| HS Code | 2934100090 |
|---|---|
| Summary | 2934100090 other compounds containing an unfused thiazole ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Gene expression of ribosomal protein mRNA inChironomus riparius: Effects of endocrine disruptor chemicals and antibiotics
Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 156(2) , 113-20, (2012) Ribosomal protein genes are essential for cellular development. To examine the effects of ribosomal protein genes under various cellular stress conditions in chironomids, ribosomal protein S3 (RpS3) a... |
|
|
DrugBank 3.0: a comprehensive resource for 'omics' research on drugs.
Nucleic Acids Res. 39 , D1035-41., (2011) DrugBank (http://www.drugbank.ca) is a richly annotated database of drug and drug target information. It contains extensive data on the nomenclature, ontology, chemistry, structure, function, action, ... |
|
|
Mechanisms of resistance to trimethoprim, the sulfonamides, and trimethoprim-sulfamethoxazole.
Rev. Infect. Dis. 4 , 261-269, (1982) A variety of different mechanisms are known to be responsible for either natural or acquired resistance to trimethoprim, the sulfonamides, or trimethoprim-sulfonamide combinations. Some mechanisms of ... |
| 2-Sulfanilamidothiazole sodium salt |
| Sodium 2-sulfanilamidothiazole |
| 4-Amino-N-(2-thiazolyl)benzenesulfonamide sodium salt |
| sulphathiazole sodium |
| MFCD00072133 |
| Monosodium 2-sulfanilamidothiazole |
| N1-2-thiazolylsulfanilamide sodium salt |
| EINECS 205-638-5 |
| Benzenesulfonamide, 4-amino-N-2-thiazolyl-, sodium salt (1:1) |
| UNII:PV16N742VM |
| Sodium [(4-aminophenyl)sulfonyl](1,3-thiazol-2-yl)azanide |
| Sulfathiazole sodium |
| Sulfathiazole (sodium) |
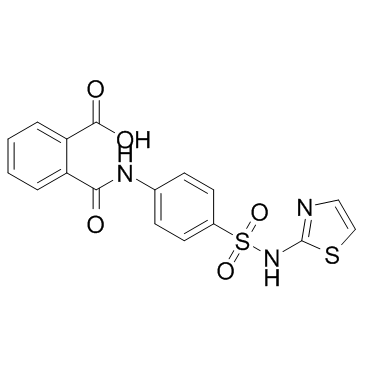 CAS#:85-73-4
CAS#:85-73-4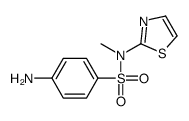 CAS#:51203-19-1
CAS#:51203-19-1