Chlojaponilactone B
Modify Date: 2025-08-25 14:16:29
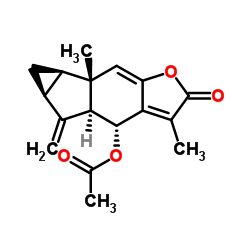
Chlojaponilactone B structure
|
Common Name | Chlojaponilactone B | ||
|---|---|---|---|---|
| CAS Number | 1449382-91-5 | Molecular Weight | 286.322 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 458.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C17H18O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 236.2±27.1 °C | |
Use of Chlojaponilactone BChlojaponilactone B is a lindenane-type sesquiterpenoid with anti-inflammatory properties. Chlojaponilactone B suppresses inflammatory responses by inhibiting TLR4 and subsequently decreasing reactive oxygen species (ROS) generation, downregulating the NF-κB, thus reducing the expression of the pro-inflammatory cytokines iNOS, NO, COX-2, IL-6 and TNF-α[1]. |
| Name | (4R,4aS,5aS,6aR,6bS)-3,6b-Dimethyl-5-methylene-2-oxo-2,4,4a,5,5a,6,6a,6b-octahydrocyclopropa[2,3]indeno[5,6-b]furan-4-yl acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Chlojaponilactone B is a lindenane-type sesquiterpenoid with anti-inflammatory properties. Chlojaponilactone B suppresses inflammatory responses by inhibiting TLR4 and subsequently decreasing reactive oxygen species (ROS) generation, downregulating the NF-κB, thus reducing the expression of the pro-inflammatory cytokines iNOS, NO, COX-2, IL-6 and TNF-α[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 458.9±45.0 °C at 760 mmHg |
| Molecular Formula | C17H18O4 |
| Molecular Weight | 286.322 |
| Flash Point | 236.2±27.1 °C |
| Exact Mass | 286.120514 |
| LogP | 2.76 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.583 |
| Storage condition | 2-8℃ |
| Cycloprop[2,3]indeno[5,6-b]furan-2(4H)-one, 4-(acetyloxy)-4a,5,5a,6,6a,6b-hexahydro-3,6b-dimethyl-5-methylene-, (4R,4aS,5aS,6aR,6bS)- |
| (4R,4aS,5aS,6aR,6bS)-3,6b-Dimethyl-5-methylene-2-oxo-2,4,4a,5,5a,6,6a,6b-octahydrocyclopropa[2,3]indeno[5,6-b]furan-4-yl acetate |