Protopanaxatriol
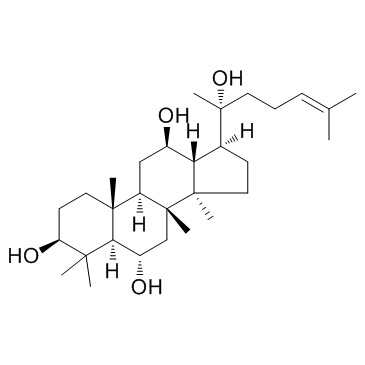
Protopanaxatriol structure
|
Common Name | Protopanaxatriol | ||
|---|---|---|---|---|
| CAS Number | 1453-93-6 | Molecular Weight | 476.732 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 590.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C30H52O4 | Melting Point | 261-263 °C | |
| MSDS | USA | Flash Point | 240.1±24.7 °C | |
Use of Protopanaxatriol20(R)-Protopanaxatriol is a natural aglycone of ginsenosides Re, Rf, Rg1, Rg2 and Rh. |
| Name | Protopanaxatriol |
|---|---|
| Synonym | More Synonyms |
| Description | 20(R)-Protopanaxatriol is a natural aglycone of ginsenosides Re, Rf, Rg1, Rg2 and Rh. |
|---|---|
| Related Catalog | |
| In Vitro | 20(R)-Protopanaxatriol is a natural aglycone of ginsenosides Re, Rf, Rg1, Rg2 and Rh[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 590.0±50.0 °C at 760 mmHg |
| Melting Point | 261-263 °C |
| Molecular Formula | C30H52O4 |
| Molecular Weight | 476.732 |
| Flash Point | 240.1±24.7 °C |
| Exact Mass | 476.386566 |
| PSA | 80.92000 |
| LogP | 5.89 |
| Vapour Pressure | 0.0±3.8 mmHg at 25°C |
| Index of Refraction | 1.541 |
| InChIKey | SHCBCKBYTHZQGZ-DLHMIPLTSA-N |
| SMILES | CC(C)=CCCC(C)(O)C1CCC2(C)C1C(O)CC1C3(C)CCC(O)C(C)(C)C3C(O)CC12C |
| Storage condition | 2-8°C |
| RIDADR | NONH for all modes of transport |
|---|
|
~93% 
Protopanaxatriol CAS#:1453-93-6 |
| Literature: Bioscience, Biotechnology and Biochemistry, , vol. 74, # 1 p. 96 - 100 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
An integrated high resolution mass spectrometric data acquisition method for rapid screening of saponins in Panax notoginseng (Sanqi).
J. Pharm. Biomed. Anal. 109 , 184-91, (2015) The aim of this study was to develop a convenient method without pretreatments for nontarget discovery of interested compounds. The segment and exposure strategy, coupled with two mass spectrometer da... |
|
|
Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis.
J. Pharm. Biomed. Anal. 99 , 8-15, (2014) American ginseng (Panax quinquefolius) is originally grown in North America. Due to price difference and supply shortage, American ginseng recently has been cultivated in northern China. Further, in t... |
|
|
Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol during ginsenoside biosynthesis in Panax ginseng.
Plant Cell Physiol. 53 , 1535-1545, (2012) Ginseng (Panax ginseng C.A. Meyer) is one of the most popular medicinal herbs, and the root of this plant contains pharmacologically active components, called ginsenosides. Ginsenosides, a class of te... |
| 20(R)-Protopanaxtriol |
| Protopanaxatriol |
| (20S)-protopanaxatriol |
| 20(R)-Protopanaxtriol(PPT) |
| Dammar-24-ene-3,6,12,20-tetrol, (3β,6α,12β,20R)- |
| (3β,6α,12β,20R)-Dammar-24-ene-3,6,12,20-tetrol |