Ginsenoside Rf
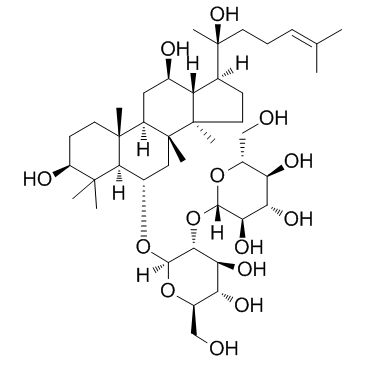
Ginsenoside Rf structure
|
Common Name | Ginsenoside Rf | ||
|---|---|---|---|---|
| CAS Number | 52286-58-5 | Molecular Weight | 801.013 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 912.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C42H72O14 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 505.5±34.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Ginsenoside RfGinsenoside Rf is a trace component of ginseng root. Ginsenoside Rf inhibits N-type Ca2+ channel. |
| Name | ginsenoside Rf |
|---|---|
| Synonym | More Synonyms |
| Description | Ginsenoside Rf is a trace component of ginseng root. Ginsenoside Rf inhibits N-type Ca2+ channel. |
|---|---|
| Related Catalog | |
| Target |
N-Type Ca2+ Channel |
| In Vitro | Ginsenoside Rf is a saponin, which is present in only trace amounts within ginseng. At saturating concentrations, Ginsenoside Rf rapidly and reversibly inhibits N-type, and other high-threshold, Ca2+ channels in rat sensory neurons to the same degree as a maximal dose of opioids. The effect is dose-dependent (half-maximal inhibition: 40 μM) and it is virtually eliminated by pretreatment of the neurons with pertussis toxin, an inhibitor of G(o) and Gi GTP-binding proteins. Ginsenoside Rf also inhibits Ca2+ channels in the hybrid F-11 cell line[1]. |
| In Vivo | Since inhibition of Ca2+ channels in sensory neurons contributes to antinociception by opioids, analgesic actions of Ginsenoside Rf are tested. Dose-dependent antinociception is found by systemic administration of Ginsenoside Rf in mice using two separate assays of tonic pain: in the acetic acid abdominal constriction test, the ED50 is 56±9 mg/kg, a concentration similar to those reported for aspirin and acetaminophen in the same assay; in the tonic phase of the biphasic formalin test, the ED50 is 129±32 mg/kg[2]. |
| Animal Admin | Mice[2] Naive, adult (7-12 week old) mice of outbred Swiss-Webster stock are used in all in vivo experiments. Mice are brought to a quiet testing room, and acclimated to table-top Plexiglas observation chambers (30 cm high; 30 cm diameter) for 30 min. They are then weighed and injected with Ginsenoside Rf (25, 50, or 75 mg/kg) or vehicle (preceded by naloxone or saline in one experiment). Twenty min later, a 0.9% solution of glacial acetic acid is injected intraperitoneally (i.p.) in a volume of 10 mL/kg. For the next 30 min, the number of constrictions (writhes)-strong contractions of the abdominal musculature accompanied by dorsoflexion of the back and extension of the hindlimbs-are counted and recorded in 5-min blocks. Four mice (one per chamber) are observed simultaneously by a single, experienced experimenter. To control for the considerable circadian and other environmental variance accompanying this nociceptive assay, two vehicle controls are tested alongside two Ginsenoside Rf-administered mice in every experimental session[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 912.3±65.0 °C at 760 mmHg |
| Molecular Formula | C42H72O14 |
| Molecular Weight | 801.013 |
| Flash Point | 505.5±34.3 °C |
| Exact Mass | 800.492188 |
| PSA | 239.22000 |
| LogP | 3.51 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.602 |
| InChIKey | UZIOUZHBUYLDHW-XUBRWZAZSA-N |
| SMILES | CC(C)=CCCC(C)(O)C1CCC2(C)C1C(O)CC1C3(C)CCC(O)C(C)(C)C3C(OC3OC(CO)C(O)C(O)C3OC3OC(CO)C(O)C(O)C3O)CC12C |
| Storage condition | ?20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 0 | |
|---|---|
| DownStream 3 | |
|
Determination of ginsenoside Rf and Rg2 from Panax ginseng using enzyme immunoassay.
Chem. Pharm. Bull. 46(7) , 1144-7, (1998) We have developed an enzyme immunoassay (EIA) to quantify trace amounts of ginsenoside Rf (Rf), one of the glycosides of protopanaxatriol from Panax ginseng. A carrier protein of bovine serum albumin ... |
|
|
[Practical optimization of the two-dimensional thin-layer chromatographic separation of ginsenosides].
Se Pu 18(6) , 546-9, (2000) A computer-assisted method is presented for optimization of mobile phase selection for separation of the mixture of ginsenosides in two-dimensional TLC. This method bases on the correlation between th... |
|
|
Microbial conversion of rare ginsenoside Rf to 20(S)-protopanaxatriol by Aspergillus niger.
Biosci. Biotechnol. Biochem. 74(1) , 96-100, (2010) In this study, rare ginsenoside Rf was transformed into 20(S)-protopanaxatriol (PPT(S)) by glycosidase from Aspergillus niger. By investing the reaction conditions, the optimal conditions were obtaine... |
| (3β,6α,12β)-3,12,20-Trihydroxydammar-24-en-6-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside |
| Ginsenoside Rf |
| GinsenosideRf |
| β-D-Glucopyranoside, (3β,6α,12β)-3,12,20-trihydroxydammar-24-en-6-yl 2-O-β-D-glucopyranosyl- |
| PANAXOSIDE RF |
| ginsinoside Rf |
 CAS#:1453-93-6
CAS#:1453-93-6 CAS#:492-61-5
CAS#:492-61-5 CAS#:80952-71-2
CAS#:80952-71-2
