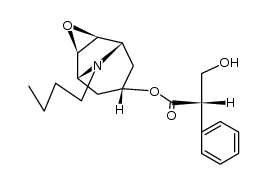
Scopolamine butylbromide
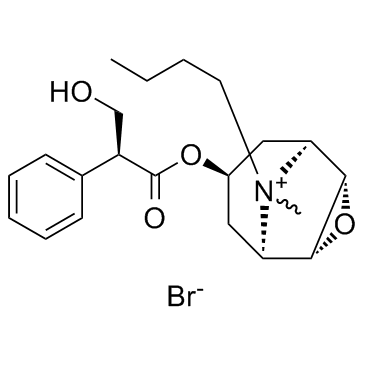
Scopolamine butylbromide structure
|
Common Name | Scopolamine butylbromide | ||
|---|---|---|---|---|
| CAS Number | 149-64-4 | Molecular Weight | 440.371 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H30BrNO4 | Melting Point | 142-1440C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Scopolamine butylbromideScopolamine butylbromide is a competitive antagonist of muscarinic acetylcholine receptor (mAChR) with an IC50 of 55.3 ± 4.3 nM.Target: mAChRScopolamine (USAN), also known as levo-duboisine and hyoscine, sold as Scopoderm, is a tropane alkaloid drug with muscarinic antagonist effects. It is among the secondary metabolites of plants from Solanaceae (nightshade) family of plants, such as henbane, jimson weed (Datura), angel's trumpets (Brugmansia), and corkwood (Duboisia). Scopolamine exerts its effects by acting as a competitive antagonist at muscarinic acetylcholine receptors, specifically M1 receptors; it is thus classified as an anticholinergic, antimuscarinic drug. Its use in medicine is relatively limited, with its chief uses being in the treatment of motion sickness and postoperative nausea and vomiting. Scopolamine is named after the plant genus Scopolia. The name "hyoscine" is from the scientific name for henbane, Hyoscyamus niger. |
| Name | N-Butylscopolammonium Bromide |
|---|---|
| Synonym | More Synonyms |
| Description | Scopolamine butylbromide is a competitive antagonist of muscarinic acetylcholine receptor (mAChR) with an IC50 of 55.3 ± 4.3 nM.Target: mAChRScopolamine (USAN), also known as levo-duboisine and hyoscine, sold as Scopoderm, is a tropane alkaloid drug with muscarinic antagonist effects. It is among the secondary metabolites of plants from Solanaceae (nightshade) family of plants, such as henbane, jimson weed (Datura), angel's trumpets (Brugmansia), and corkwood (Duboisia). Scopolamine exerts its effects by acting as a competitive antagonist at muscarinic acetylcholine receptors, specifically M1 receptors; it is thus classified as an anticholinergic, antimuscarinic drug. Its use in medicine is relatively limited, with its chief uses being in the treatment of motion sickness and postoperative nausea and vomiting. Scopolamine is named after the plant genus Scopolia. The name "hyoscine" is from the scientific name for henbane, Hyoscyamus niger. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 142-1440C |
|---|---|
| Molecular Formula | C21H30BrNO4 |
| Molecular Weight | 440.371 |
| Exact Mass | 439.135803 |
| PSA | 59.06000 |
| Water Solubility | H2O: 50 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | YM3500000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2939999090 |
|
~% 
Scopolamine but... CAS#:149-64-4 |
| Literature: GB708370 , ; DE856890 , ; |
|
~% 
Scopolamine but... CAS#:149-64-4 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 35, # 1 A p. 217 - 228 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn's disease: correlation with intraoperative findings and magnetic resonance enterography.
J. Gastroenterol. Hepatol. 30(1) , 86-91, (2014) In evaluating small bowel Crohn's disease (CD), small intestine contrast-enhanced ultrasonography (SICUS) is emerging as an alternative to magnetic resonance enterography (MRE). This retrospective stu... |
|
|
Lymphoid nodular hyperplasia of the terminal ileum can mimic active crohn disease on MR enterography.
AJR Am. J. Roentgenol. 203(4) , W400-7, (2014) The purpose of this article is to describe the MRI findings associated with lymphoid nodular hyperplasia at MR enterography and test the ability of radiologists to differentiate healthy control subjec... |
| (1R,2R,4S,5S,7s,9S)-9-Butyl-7-{[(2S)-3-hydroxy-2-phenylpropanoyl]oxy}-9-methyl-3-oxa-9-azoniatricyclo[3.3.1.0]nonane bromide |
| (−)-N-Butylscopolamine bromide (−)-Scopolamine N-butyl bromide Hyoscine N-butyl bromide |
| EINECS 205-744-1 |
| MFCD00078561 |
| 3-Oxa-9-azoniatricyclo[3.3.1.0]nonane, 9-butyl-7-[(2S)-3-hydroxy-1-oxo-2-phenylpropoxy]-9-methyl-, bromide, (1R,2R,4S,5S)- (1:1) |
| Scopolamine butylbromide |
| Hyoscine butylbromide |
| Hyoscinen buty1bromide |