Ac-Asp-Tyr(PO3H2)-Val-Pro-Met-Leu-NH2
Modify Date: 2024-01-07 22:37:35
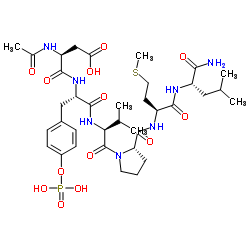
Ac-Asp-Tyr(PO3H2)-Val-Pro-Met-Leu-NH2 structure
|
Common Name | Ac-Asp-Tyr(PO3H2)-Val-Pro-Met-Leu-NH2 | ||
|---|---|---|---|---|
| CAS Number | 157382-69-9 | Molecular Weight | 857.908 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C36H56N7O13PS | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Ac-Asp-Tyr(PO3H2)-Val-Pro-Met-Leu-NH2Ac-Asp-Tyr(PO3H2)-Val-Pro-Met-Leu-NH2 is the SH2 domain ligand. SH2 domains participate in protein tyrosine kinase (PTK)-mediated cellular signal[1]. |
| Name | N-Acetyl-L-α-aspartyl-O-phosphono-L-tyrosyl-L-valyl-L-prolyl-L-methionyl-L-leucinamide |
|---|---|
| Synonym | More Synonyms |
| Description | Ac-Asp-Tyr(PO3H2)-Val-Pro-Met-Leu-NH2 is the SH2 domain ligand. SH2 domains participate in protein tyrosine kinase (PTK)-mediated cellular signal[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C36H56N7O13PS |
| Molecular Weight | 857.908 |
| Exact Mass | 857.339417 |
| LogP | 0.55 |
| Index of Refraction | 1.572 |
| N-Acetyl-L-α-aspartyl-O-phosphono-L-tyrosyl-L-valyl-L-prolyl-L-methionyl-L-leucinamide |
| L-Leucinamide, N-acetyl-L-α-aspartyl-O-phosphono-L-tyrosyl-L-valyl-L-prolyl-L-methionyl- |