Nelfinavir
Modify Date: 2024-01-05 13:25:57

Nelfinavir structure
|
Common Name | Nelfinavir | ||
|---|---|---|---|---|
| CAS Number | 159989-64-7 | Molecular Weight | 567.78 | |
| Density | 1.22g/cm3 | Boiling Point | 786.8ºC at 760 mmHg | |
| Molecular Formula | C32H45N3O4S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 429.7ºC | |
Use of NelfinavirNelfinavir(AG-1341) is a potent and orally bioavailable human immunodeficiency virus HIV-1 protease inhibitor (Ki=2 nM) and is widely prescribed in combination with HIV reverse transcriptase inhibitors for the treatment of HIV infection. IC50 Valur: 2 nM (Ki for HIV-1 protease) [2]Target: HIV Proteasein vitro: In vitro exposure (72 hours) of HAECs to NEL (0.25-2 μg/mL) decreased both basal (2.5-fold) and insulin-induced NO production (4- to 5-fold). NEL suppressed insulin-induced phosphorylation of both Akt and eNOS at serine residues 473 and 1177, respectively. NEL decreased tyrosine phosphorylation of IR-β, IRS-1, and PI3K. Coexposure to troglitazone (TRO; 250 nM) ameliorated the suppressive effects of NEL on insulin signaling and NO production. Coexposure to TRO also increased eNOS expression in NEL-treated HAECs [1]. AG1343 is a potent enzyme inhibitor (Ki = 2 nM) and antiviral agent (HIV-1 ED50 = 14 nM). An X-ray cocrystal structure of the enzyme-AG1343 complex reveals how the novel thiophenyl ether and phenol-amide substituents of the inhibitor interact with the S1 and S2 subsites of HIV-1 protease, respectively [2].in vivo: In vivo studies indicate that AG1343 is well absorbed orally in a variety of species and possesses favorable pharmacokinetic properties in humans [2]. |
| Name | nelfinavir |
|---|---|
| Synonym | More Synonyms |
| Description | Nelfinavir(AG-1341) is a potent and orally bioavailable human immunodeficiency virus HIV-1 protease inhibitor (Ki=2 nM) and is widely prescribed in combination with HIV reverse transcriptase inhibitors for the treatment of HIV infection. IC50 Valur: 2 nM (Ki for HIV-1 protease) [2]Target: HIV Proteasein vitro: In vitro exposure (72 hours) of HAECs to NEL (0.25-2 μg/mL) decreased both basal (2.5-fold) and insulin-induced NO production (4- to 5-fold). NEL suppressed insulin-induced phosphorylation of both Akt and eNOS at serine residues 473 and 1177, respectively. NEL decreased tyrosine phosphorylation of IR-β, IRS-1, and PI3K. Coexposure to troglitazone (TRO; 250 nM) ameliorated the suppressive effects of NEL on insulin signaling and NO production. Coexposure to TRO also increased eNOS expression in NEL-treated HAECs [1]. AG1343 is a potent enzyme inhibitor (Ki = 2 nM) and antiviral agent (HIV-1 ED50 = 14 nM). An X-ray cocrystal structure of the enzyme-AG1343 complex reveals how the novel thiophenyl ether and phenol-amide substituents of the inhibitor interact with the S1 and S2 subsites of HIV-1 protease, respectively [2].in vivo: In vivo studies indicate that AG1343 is well absorbed orally in a variety of species and possesses favorable pharmacokinetic properties in humans [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.22g/cm3 |
|---|---|
| Boiling Point | 786.8ºC at 760 mmHg |
| Molecular Formula | C32H45N3O4S |
| Molecular Weight | 567.78 |
| Flash Point | 429.7ºC |
| PSA | 189.95000 |
| LogP | 6.05210 |
| Vapour Pressure | 4.38E-26mmHg at 25°C |
| Index of Refraction | 1.618 |
| Storage condition | 2-8℃ |
| Methansulfonsäure--(3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-{[(3-hydroxy-2-methylphenyl)carbonyl]amino}-4-(phenylsulfanyl)butyl]decahydroisochinolin-3-carboxamid(1:1) |
| Nelfinavir mesylate |
| Viracept (TN) |
| (3S,4aS,8aS)-N-tert-Butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylsulfanyl)butyl]decahydroisoquinoline-3-carboxamide methanesulfonate (1:1) |
| Nelfinavir (INN) |
| Nelfinavir Monomethane Sulfonate |
| Viracept |
| acide méthanesulfonique - (3S,4aS,8aS)-N-(1,1-diméthyléthyl)-2-[(2R,3R)-2-hydroxy-3-{[(3-hydroxy-2-méthylphényl)carbonyl]amino}-4-(phénylsulfanyl)butyl]décahydroisoquinoléine-3-carboxamide (1:1) |
| AG1343 |
| (3S,4AS,8AS)-2-[(2R,3R)-2-hydroxy-3-(3-hydroxy-2-methylbenzoylamino)-4-phenylthiobutyl]decahydroisoquinoline-3-carboxylic acid t-butylamide |
| 3-Isoquinolinecarboxamide, N-(1,1-dimethylethyl)decahydro-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-, (3S,4aS,8aS)-, methanesulfonate (1:1) (salt) |
| (3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]decahydroisoquinoline-3-carboxamide methanesulfonate (salt) |
| [3H]-Nelfinavir |
| UNII-HO3OGH5D7I |
| (3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-{[(3-hydroxy-2-methylphenyl)carbonyl]amino}-4-(phenylsulfanyl)butyl]decahydroisoquinoline-3-carboxamide methanesulfonate (salt) |
| (3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinoline-3-carboxamide |
| VRX496 |
| [14C]-Nelfinavir |
| AZT/3TC/NLFR combination |
| (3S-(2(2S*,3S*),3-a,4ab,8ab))-N-(1,1-dimethylethyl)decahydro-2-(2-hydroxy-3-((3-hydroxy-2-methylbenzoyl)amino)-4-(phenylthio)butyl)-3-isoquinolinecarboxamide monomethanesulfonate (salt) |
| (3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylsulfanyl)butyl]-N-(2-methyl-2-propanyl)decahydro-3-isoquinolinecarboxamide methanesulfonate (1:1) |
| [3S-(3R*,4AR*,8AR*,2'S*,3'S*)]-2-[2'-Hydroxy-3'-phenylthiomethyl-4'-aza-5'-oxo-5'-(2''-methyl-3''-hydroxyphenyl)pentyl]decahydroisoquinoline-3-N-t-butylcarboxamide |
| 1ohr |
 CAS#:108-98-5
CAS#:108-98-5 CAS#:188936-07-4
CAS#:188936-07-4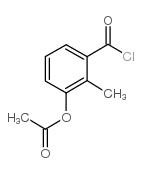 CAS#:167678-46-8
CAS#:167678-46-8 CAS#:128019-40-9
CAS#:128019-40-9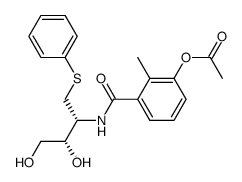 CAS#:767341-27-5
CAS#:767341-27-5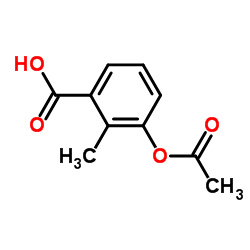 CAS#:168899-58-9
CAS#:168899-58-9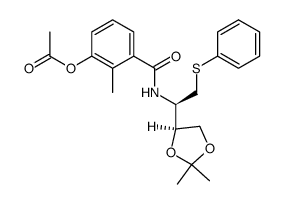 CAS#:767341-31-1
CAS#:767341-31-1 CAS#:159989-65-8
CAS#:159989-65-8