Z-Ile-Leu-aldehyde
Modify Date: 2025-08-25 12:17:32
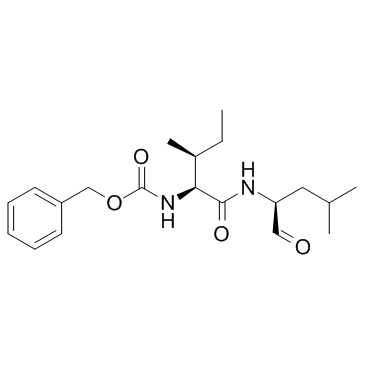
Z-Ile-Leu-aldehyde structure
|
Common Name | Z-Ile-Leu-aldehyde | ||
|---|---|---|---|---|
| CAS Number | 161710-10-7 | Molecular Weight | 362.46300 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C20H30N2O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Z-Ile-Leu-aldehydeZ-Ile-Leu-aldehyde(Z-IL-CHO; GSI-XII) is a potent gamma-Secretase inhibitor; Notch signaling inhibitor.IC50 value:Target: gamma-Secretase inhibitorin vitro: GSI-XII induces apoptosis of murine MOPC315.BM myeloma cells with high Notch activity. GSI XII impairs murine osteoclast differentiation of receptor activator of NF-κB ligand (RANKL)-stimulated RAW264.7 cells in vitro [1]. Notch-signaling inhibition in HRS cells by the γ-secretase inhibitor (GSI) XII results in decreased alternative p52/RelB NF-κB signaling, interfering with processing of the NF-κB2 gene product p100 into its active form p52 [2]. GSI treatment induced morphologic erythroid differentiation and promoted hemoglobin production. GSI treatment suppressed short-term growth and colony formation, while treatment with GSI-XXI promoted the growth of AA cells [3].in vivo: In the murine MOPC315.BM myeloma model GSI XII has potent anti-MM activity and reduces osteolytic lesions as evidenced by diminished myeloma-specific monoclonal immunoglobulin (Ig)-A serum levels and quantitative assessment of bone structure changes via high-resolution microcomputed tomography scans [1]. |
| Name | Z-Ile-Leu-aldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | Z-Ile-Leu-aldehyde(Z-IL-CHO; GSI-XII) is a potent gamma-Secretase inhibitor; Notch signaling inhibitor.IC50 value:Target: gamma-Secretase inhibitorin vitro: GSI-XII induces apoptosis of murine MOPC315.BM myeloma cells with high Notch activity. GSI XII impairs murine osteoclast differentiation of receptor activator of NF-κB ligand (RANKL)-stimulated RAW264.7 cells in vitro [1]. Notch-signaling inhibition in HRS cells by the γ-secretase inhibitor (GSI) XII results in decreased alternative p52/RelB NF-κB signaling, interfering with processing of the NF-κB2 gene product p100 into its active form p52 [2]. GSI treatment induced morphologic erythroid differentiation and promoted hemoglobin production. GSI treatment suppressed short-term growth and colony formation, while treatment with GSI-XXI promoted the growth of AA cells [3].in vivo: In the murine MOPC315.BM myeloma model GSI XII has potent anti-MM activity and reduces osteolytic lesions as evidenced by diminished myeloma-specific monoclonal immunoglobulin (Ig)-A serum levels and quantitative assessment of bone structure changes via high-resolution microcomputed tomography scans [1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C20H30N2O4 |
|---|---|
| Molecular Weight | 362.46300 |
| Exact Mass | 362.22100 |
| PSA | 84.50000 |
| LogP | 3.83910 |
| Storage condition | 2-8℃ |
| z-il-cho |