H-Gly-NH2.HCl
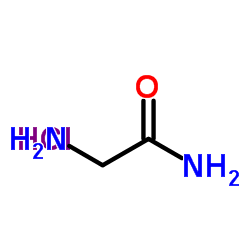
H-Gly-NH2.HCl structure
|
Common Name | H-Gly-NH2.HCl | ||
|---|---|---|---|---|
| CAS Number | 1668-10-6 | Molecular Weight | 110.54 | |
| Density | 1.122g/cm3 | Boiling Point | 281.3ºC at 760mmHg | |
| Molecular Formula | C2H7ClN2O | Melting Point | 204 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of H-Gly-NH2.HCl2-Aminoacetamide hydrochloride is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Glycinamide hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Aminoacetamide hydrochloride is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.122g/cm3 |
|---|---|
| Boiling Point | 281.3ºC at 760mmHg |
| Melting Point | 204 °C (dec.)(lit.) |
| Molecular Formula | C2H7ClN2O |
| Molecular Weight | 110.54 |
| Exact Mass | 110.024689 |
| PSA | 69.11000 |
| LogP | 0.63300 |
| Vapour Pressure | 0.00359mmHg at 25°C |
| Storage condition | Store at RT. |
| Water Solubility | H2O: 0.1 g/mL, clear |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25-S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29241900 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Preparation of guanine and diaminopurine from biuret. Part III.
Chem. Biodivers. 4(4) , 818-22, (2007) Because of their potential prebiotic origin and relative chemical stability, urea, biuret, formic acid, and glycine amide might have played a role in the assembly process of purine bases. In this pape... |
|
|
Identification of quinoline, carboline and glycinamide compounds in cow milk using HRMS and NMR.
Food Chem. 141(3) , 1888-94, (2013) The aim of this work was to characterise new UV-absorbing compounds (UAC) in cow milk in order to gain an overview of the molecular diversity of the minor bioactive constituents, that could be used to... |
|
|
Synthesis of (-)-quinocarcin by directed condensation of alpha-amino aldehydes.
J. Am. Chem. Soc. 127(48) , 16796-7, (2005) An enantioselective synthesis of the natural antiproliferative agent quinocarcin was achieved by the directed condensation of optically active alpha-amino aldehyde intermediates. Condensation of the N... |
| Acetamide, 2-amino-, monohydrochloride |
| 2-Aminoacetamide hydrochloride |
| Glycinamide hydrochloride (1:1) |
| MFCD00013008 |
| Glycine amide hydrochloride |
| Glycinamide, monohydrochloride |
| EINECS 216-789-1 |
| 2-aminoacetamide,hydrochloride |
| Acetamide, 2-amino-, hydrochloride (1:1) |
| H-Gly-NH2.HCl |
| Glycinamidehydrochloride |
 CAS#:540-61-4
CAS#:540-61-4 CAS#:5680-79-5
CAS#:5680-79-5![1-Oxa-6,9-diazaspiro[4.5]decane-7,10-dione,4-methylene-(9CI) Structure](https://image.chemsrc.com/caspic/176/141509-41-3.png) CAS#:141509-41-3
CAS#:141509-41-3 CAS#:39796-49-1
CAS#:39796-49-1 CAS#:35150-09-5
CAS#:35150-09-5 CAS#:949-90-6
CAS#:949-90-6 CAS#:25844-72-8
CAS#:25844-72-8 CAS#:2479-62-1
CAS#:2479-62-1 CAS#:20721-18-0
CAS#:20721-18-0 CAS#:20721-17-9
CAS#:20721-17-9 CAS#:598-41-4
CAS#:598-41-4 CAS#:6422-35-1
CAS#:6422-35-1 CAS#:62613-82-5
CAS#:62613-82-5
