(4-Chlorophenyl)boronic acid
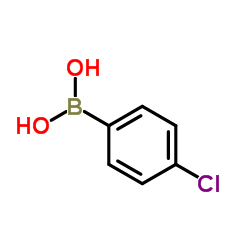
(4-Chlorophenyl)boronic acid structure
|
Common Name | (4-Chlorophenyl)boronic acid | ||
|---|---|---|---|---|
| CAS Number | 1679-18-1 | Molecular Weight | 156.375 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 295.4±42.0 °C at 760 mmHg | |
| Molecular Formula | C6H6BClO2 | Melting Point | 284-289 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 132.4±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 4-Chlorophenylboronic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 295.4±42.0 °C at 760 mmHg |
| Melting Point | 284-289 °C(lit.) |
| Molecular Formula | C6H6BClO2 |
| Molecular Weight | 156.375 |
| Flash Point | 132.4±27.9 °C |
| Exact Mass | 156.014938 |
| PSA | 40.46000 |
| LogP | 2.18 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.558 |
| InChIKey | CAYQIZIAYYNFCS-UHFFFAOYSA-N |
| SMILES | OB(O)c1ccc(Cl)cc1 |
| Storage condition | Refrigerator (+4°C) |
| Water Solubility | 2.5 g/100 mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S36-S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CY8950000 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
|
Cationic Pd(II)-catalyzed highly enantioselective arylative cyclization of alkyne-tethered enals or enones initiated by carbopalladation of alkynes with arylboronic acids.
Org. Lett. 7th ed., 14 , 1756-1759, (2012) Cationic Pd(II)-catalyzed enantioselective arylative cyclization of alkyne-tethered enals or enones initiated by carbopalladation of alkynes was developed without the necessity of a redox system. |
|
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combin... |
|
|
Tandem-type Pd(II)-catalyzed oxidative Heck reaction/intramolecular C-H amidation sequence: a novel route to 4-aryl-2-quinolinones.
Chem. Commun. (Camb.) 36th ed., 48 , 4332-4334, (2012) A novel catalytic method for synthesizing 4-aryl-2-quinolinones is reported. The process involves two mechanistically independent, sequential Pd(II)-catalyzed reactions--the oxidative Heck reaction an... |
| 4-Chlorophenylboranic acid |
| p-chlorophenylboronic aci |
| para-chlorophenylboronic acid |
| (4-Chlorophenyl)boronic acid |
| para-chlorobenzene boronic acid |
| 4-CHLOROPHENYLBORNIC ACID |
| p-chloro-benzeneboronicaci |
| MFCD00039137 |
| boronicacid,p-chlorophenyl |
| 4-Chlorophenylboronic acid |
| EINECS 216-845-5 |
| 4-chlorobenzeneboronic acid |
| 4-Chloro phenyl boronic acid |
| p-chlorophenylboronic acid |
| p-chlorobenzeneboronic acid |
| RARECHEM AH PB 0178 |
| Boronic acid, B-(4-chlorophenyl)- |
 CAS#:29540-84-9
CAS#:29540-84-9 CAS#:106-39-8
CAS#:106-39-8 CAS#:5419-55-6
CAS#:5419-55-6 CAS#:688-71-1
CAS#:688-71-1 CAS#:13283-31-3
CAS#:13283-31-3 CAS#:873-77-8
CAS#:873-77-8 CAS#:7637-07-2
CAS#:7637-07-2 CAS#:120208-73-3
CAS#:120208-73-3 CAS#:7732-18-5
CAS#:7732-18-5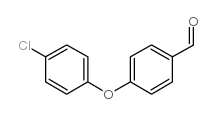 CAS#:61343-99-5
CAS#:61343-99-5 CAS#:3506-75-0
CAS#:3506-75-0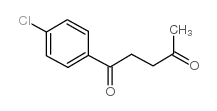 CAS#:53842-12-9
CAS#:53842-12-9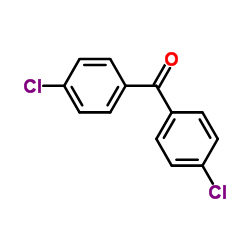 CAS#:90-98-2
CAS#:90-98-2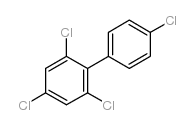 CAS#:32598-12-2
CAS#:32598-12-2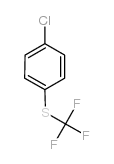 CAS#:407-16-9
CAS#:407-16-9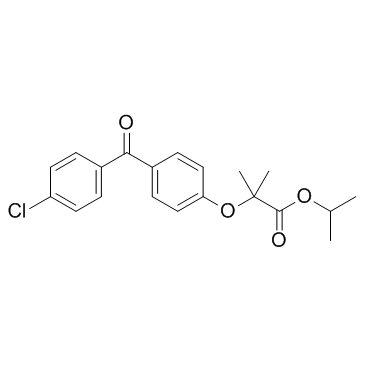 CAS#:49562-28-9
CAS#:49562-28-9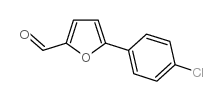 CAS#:34035-03-5
CAS#:34035-03-5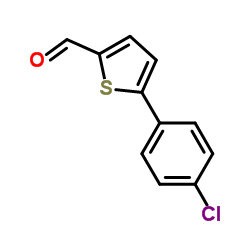 CAS#:38401-71-7
CAS#:38401-71-7 CAS#:5748-41-4
CAS#:5748-41-4
