H-Asp(OMe)-OH·HCl
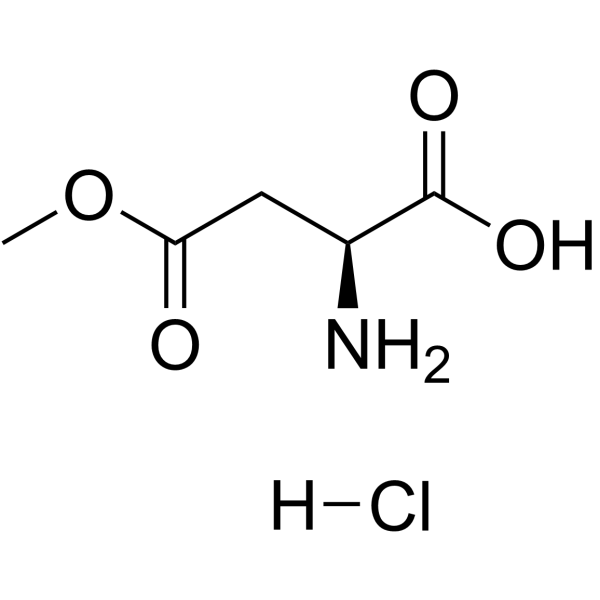
H-Asp(OMe)-OH·HCl structure
|
Common Name | H-Asp(OMe)-OH·HCl | ||
|---|---|---|---|---|
| CAS Number | 16856-13-6 | Molecular Weight | 183.590 | |
| Density | 1.299g/cm3 | Boiling Point | 301.7ºC at 760mmHg | |
| Molecular Formula | C5H10ClNO4 | Melting Point | 191-193°C | |
| MSDS | Chinese USA | Flash Point | 136.3ºC | |
Use of H-Asp(OMe)-OH·HClβ-Methyl L-aspartate hydrochloride is an aspartic acid derivative[1]. |
| Name | L-Aspartic acid β-methyl ester hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | β-Methyl L-aspartate hydrochloride is an aspartic acid derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.299g/cm3 |
|---|---|
| Boiling Point | 301.7ºC at 760mmHg |
| Melting Point | 191-193°C |
| Molecular Formula | C5H10ClNO4 |
| Molecular Weight | 183.590 |
| Flash Point | 136.3ºC |
| Exact Mass | 183.029831 |
| PSA | 89.62000 |
| LogP | 0.46370 |
| Vapour Pressure | 0.000242mmHg at 25°C |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2922509090 |
|
~96% 
H-Asp(OMe)-OH·HCl CAS#:16856-13-6 |
| Literature: Baldwin, Jack E.; Moloney, Mark G.; North, Michael Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1989 , p. 833 - 834 |
|
~99% 
H-Asp(OMe)-OH·HCl CAS#:16856-13-6 |
| Literature: Gmeiner; Feldman; Chu-Moyer; Rapoport Journal of Organic Chemistry, 1990 , vol. 55, # 10 p. 3068 - 3074 |
|
~% 
H-Asp(OMe)-OH·HCl CAS#:16856-13-6 |
| Literature: WO2010/105367 A1, ; Page/Page column 35; 36; 37 ; |
|
~77% 
H-Asp(OMe)-OH·HCl CAS#:16856-13-6 |
| Literature: Gmeiner; Feldman; Chu-Moyer; Rapoport Journal of Organic Chemistry, 1990 , vol. 55, # 10 p. 3068 - 3074 |
| Precursor 4 | |
|---|---|
| DownStream 9 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Syntheses of S-enantiomers of hanishin, longamide B, and longamide B methyl ester from L-aspartic acid beta-methyl ester: establishment of absolute stereochemistry.
J. Org. Chem. 70 , 9081-9084, (2005) [reaction: see text] Total syntheses of enantiopure hanishin, longamide B, and longamide B methyl ester are described. Absolute configurations of these natural products have been established. |
|
|
Enzymatic synthesis of a CCK-4 tripeptide fragment.
Di Yi Jun Yi Da Xue Xue Bao 23 , 289-292, (2003) To synthesize a tripeptide derivative Phac-Met-Asp(OMe)-Phe -NH2, which is a fragment of the gastrin C-terminal tetrapeptide CCK-4, by enzymatic reaction.Three free enzymes, alpha-chymotrypsin, papain... |
|
|
Practical synthesis of (R)-4-mercaptopyrrolidine-2-thione from L-aspartic acid. Preparation of a novel orally active 1-beta-methylcarbapenem, TA-949.
J. Org. Chem. 65 , 517-522, (2000) A facile and economical synthesis of a novel orally active 1-beta-methylcarbapenem, TA-949 (1), is described. The key process involves an efficient synthesis of the C-2 side chain (R)-4-mercaptopyrrol... |
| (2S)-2-Amino-4-methoxy-4-oxobutanoic acid hydrochloride (1:1) |
| L-Aspartatic Acid 4-Methyl Ester Hydrochloride |
| 4-Methyl L-Aspartate Hydrochloride |
| EINECS 240-880-5 |
| L-Asparitc acid 4-methyl ester HCl |
| L-Aspartic acid, 4-methyl ester, hydrochloride (1:1) |
| MFCD00038972 |
| H-Asp(OMe)-OH.HCl |
| H-Asp(OMe)-OH·HCl |
| (S)-2-Amino-4-methoxy-4-oxobutanoic acid hydrochloride |
| beta-Methyl L-aspartate hydrochloride |
| β-Methyl L-aspartate hydrochloride |

 CAS#:3160-47-2
CAS#:3160-47-2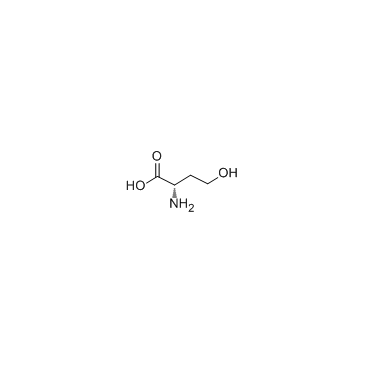 CAS#:672-15-1
CAS#:672-15-1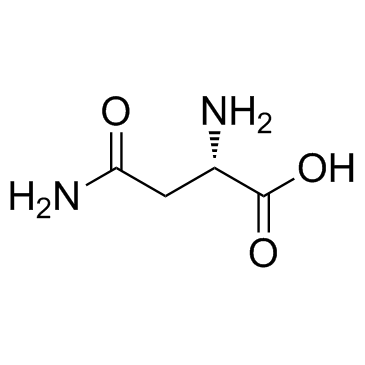 CAS#:70-47-3
CAS#:70-47-3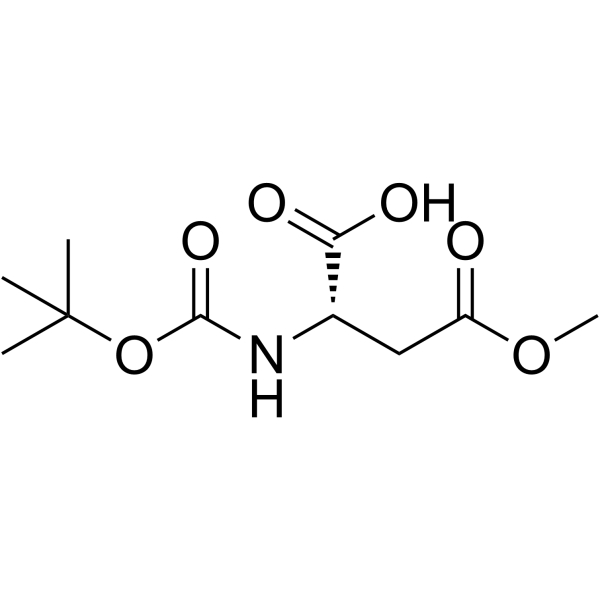 CAS#:59768-74-0
CAS#:59768-74-0 CAS#:145038-53-5
CAS#:145038-53-5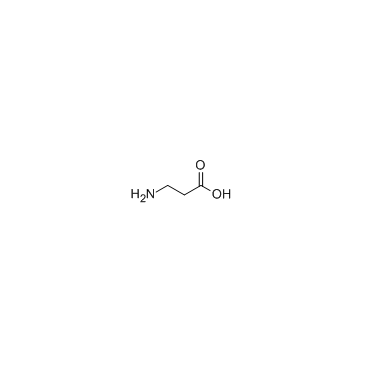 CAS#:107-95-9
CAS#:107-95-9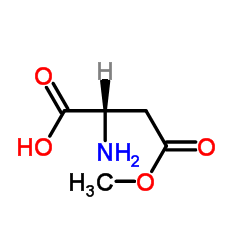 CAS#:2177-62-0
CAS#:2177-62-0![L-Aspartic acid,N-[N-[(phenylmethoxy)carbonyl]glycyl]-, 4-methyl ester (9CI) structure](https://image.chemsrc.com/caspic/091/6120-55-4.png) CAS#:6120-55-4
CAS#:6120-55-4 CAS#:39741-26-9
CAS#:39741-26-9
