Zearalenone
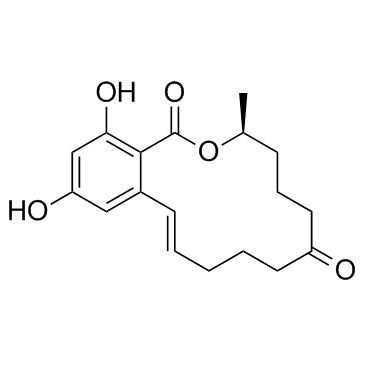
Zearalenone structure
|
Common Name | Zearalenone | ||
|---|---|---|---|---|
| CAS Number | 17924-92-4 | Molecular Weight | 318.364 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 600.4±55.0 °C at 760 mmHg | |
| Molecular Formula | C18H22O5 | Melting Point | 164-165°C | |
| MSDS | Chinese USA | Flash Point | 219.5±25.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of ZearalenoneZearalenone is a mycotoxin produced mainly by fungi belonging to the genus Fusarium in foods and feeds. Possess oestrogenic activity in pigs, cattle and sheep, with low acute toxicity. Causes precocious development of mammae and other estrogenic effects in young gilts[1][2]. |
| Name | zearalenone |
|---|---|
| Synonym | More Synonyms |
| Description | Zearalenone is a mycotoxin produced mainly by fungi belonging to the genus Fusarium in foods and feeds. Possess oestrogenic activity in pigs, cattle and sheep, with low acute toxicity. Causes precocious development of mammae and other estrogenic effects in young gilts[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 600.4±55.0 °C at 760 mmHg |
| Melting Point | 164-165°C |
| Molecular Formula | C18H22O5 |
| Molecular Weight | 318.364 |
| Flash Point | 219.5±25.0 °C |
| Exact Mass | 318.146729 |
| PSA | 83.83000 |
| LogP | 3.83 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.539 |
| InChIKey | MBMQEIFVQACCCH-QBODLPLBSA-N |
| SMILES | CC1CCCC(=O)CCCC=Cc2cc(O)cc(O)c2C(=O)O1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H312 + H332-H319 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C:Corrosive; |
| Risk Phrases | R34;R62;R63 |
| Safety Phrases | S26-S36/37/39-S45-S36-S16-S36/37 |
| RIDADR | UN 3261 8/PG 2 |
| WGK Germany | 3 |
| RTECS | DM2550000 |
| Packaging Group | II; III |
| Hazard Class | 4.1 |
| Precursor 5 | |
|---|---|
| DownStream 6 | |
|
Deoxynivalenol-mimic nanobody isolated from a naïve phage display nanobody library and its application in immunoassay.
Anal. Chim. Acta 887 , 201-8, (2015) In this study, using mycotoxin deoxynivalenol (DON) as a model hapten, we developed a nanobody-based environmental friendly immunoassay for sensitive detection of DON. Two nanobodies (N-28 and N-31) w... |
|
|
Mitochondrial proteomic analysis reveals the molecular mechanisms underlying reproductive toxicity of zearalenone in MLTC-1 cells.
Toxicology 324 , 55-67, (2014) Zearalenone (ZEA), a Fusarium mycotoxin that contaminates cereal crops worldwide, has been shown to affect the male reproductive system and trigger reactive oxygen species (ROS) generation. However, t... |
|
|
The effects of pH and surfactants on the absorption and fluorescence properties of ochratoxin A and zearalenone.
Luminescence 30 , 1106-11, (2015) The pH and surfactant dependencies of the absorption and fluorescence properties of ochratoxin A (OTA) and zearalenone (ZEN), the main mycotoxins found as contaminants in foods and feeds, were evaluat... |
| (S)-(-)-Zearalenone |
| (3S,11E)-3,4,5,6,9,10-Hexahydro-14,16-dihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1,7(8H)-dione |
| (S)-Zearalenone |
| 6-(10-Hydroxy-6-oxo-trans-1-undecenyl)-b-resorcylic Acid Lactone |
| MFCD00133085 |
| 1H-2-Benzoxacyclotetradecin-1,7(8H)-dione, 3,4,5,6,9,10-hexahydro-14,16-dihydroxy-3-methyl-, (3S,11E)- |
| (-)-Zearalenone |
| Zearalenone |
| EINECS 241-864-0 |
| (3S,11E)-14,16-Dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dione |
 CAS#:64584-92-5
CAS#:64584-92-5 CAS#:928-90-5
CAS#:928-90-5 CAS#:2150-47-2
CAS#:2150-47-2 CAS#:73448-13-2
CAS#:73448-13-2![(R)-4-[(tert-butyldimethylsilyl)oxy]-1-pentanol Structure](https://image.chemsrc.com/caspic/299/104784-04-5.png) CAS#:104784-04-5
CAS#:104784-04-5 CAS#:105088-14-0
CAS#:105088-14-0 CAS#:36455-72-8
CAS#:36455-72-8 CAS#:71030-11-0
CAS#:71030-11-0 CAS#:5975-78-0
CAS#:5975-78-0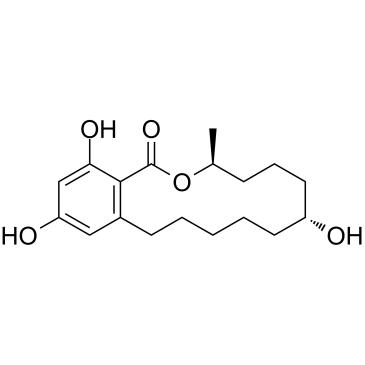 CAS#:26538-44-3
CAS#:26538-44-3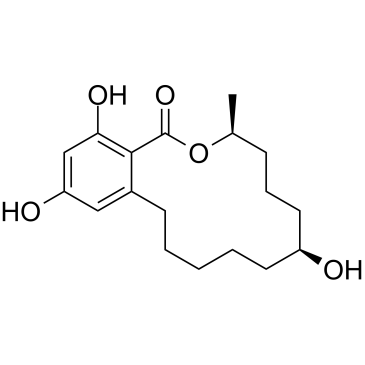 CAS#:42422-68-4
CAS#:42422-68-4
