Dacisteine

Dacisteine structure
|
Common Name | Dacisteine | ||
|---|---|---|---|---|
| CAS Number | 18725-37-6 | Molecular Weight | 205.23200 | |
| Density | 1.314g/cm3 | Boiling Point | 446.8ºC at 760 mmHg | |
| Molecular Formula | C7H11NO4S | Melting Point | 114-116°C | |
| MSDS | N/A | Flash Point | 224ºC | |
Use of DacisteineDacisteine (N,S-Diacetyl-L-cysteine) is a cysteine derivative and displays a less New Delhi metallo-beta-lactamase-1 (NDM-1) inhibitor with an IC50 value of 1000 μM[1]. Dacisteine can be used for the treatment of cardiovascular and cerebrovascular diseases caused by platelet aggregation[2]. |
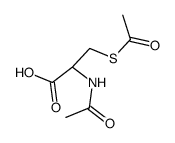
| Name | (2R)-2-acetamido-3-acetylsulfanylpropanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Dacisteine (N,S-Diacetyl-L-cysteine) is a cysteine derivative and displays a less New Delhi metallo-beta-lactamase-1 (NDM-1) inhibitor with an IC50 value of 1000 μM[1]. Dacisteine can be used for the treatment of cardiovascular and cerebrovascular diseases caused by platelet aggregation[2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 1000 μM (NDM-1)[1] |
| In Vivo | Dacisteine is a preparing agent for treating or preventing cardiovascular and cerebrovascular diseases caused by platelet aggregation. |
| References |
| Density | 1.314g/cm3 |
|---|---|
| Boiling Point | 446.8ºC at 760 mmHg |
| Melting Point | 114-116°C |
| Molecular Formula | C7H11NO4S |
| Molecular Weight | 205.23200 |
| Flash Point | 224ºC |
| Exact Mass | 205.04100 |
| PSA | 108.77000 |
| LogP | 0.24630 |
| Vapour Pressure | 3.13E-09mmHg at 25°C |
| Index of Refraction | 1.522 |
| Storage condition | Refrigerator |
| HS Code | 2930909090 |
|---|
|
~81% 
Dacisteine CAS#:18725-37-6 |
| Literature: Pharmazie, , vol. 49, # 4 p. 249 - 252 |
|
~% 
Dacisteine CAS#:18725-37-6 |
| Literature: Farmaco, Edizione Scientifica, , vol. 31, # 11 p. 767 - 775 |
|
~% 
Dacisteine CAS#:18725-37-6 |
| Literature: Biochemical Journal, , vol. 57, p. 360,363 |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Formation of the thioester, N,S-diacetylcysteine, from acetaldehyde and N,N'diacetylcystine in aqueous solution with ultraviolet light.
J. Mol. Evol. 17(2) , 103-7, (1981) The thioester, N,S-diacetylcysteine, is formed during the illumination of phosphate buffered (pH 7.0) aqueous solutions of acetaldehyde and N,N'-diacetylcystine with ultraviolet light. The yield of N,... |
| Dacisteina |
| Dacisteine |
| S,N-diacetylcysteine |
| N,S-Diacetyl |
| Acetylcysteine impurity D |
| N,S-diacetyl-D,L-cysteine |
| N,S-diacetyl-L-cysteine |
| N,S-Diacetylcysteine |
| Dacisteinum |
| N,S-diacetil-L-cisteina |


 CAS#:1078-38-2
CAS#:1078-38-2
