Darunavir
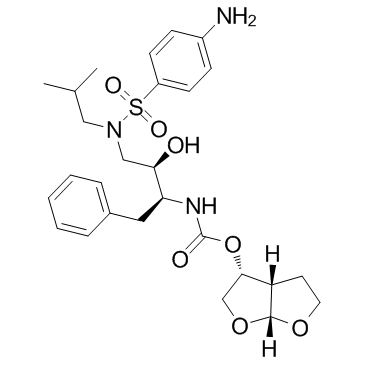
Darunavir structure
|
Common Name | Darunavir | ||
|---|---|---|---|---|
| CAS Number | 206361-99-1 | Molecular Weight | 547.664 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C27H37N3O7S | Melting Point | 74-76ºC | |
| MSDS | N/A | Flash Point | N/A | |
Use of DarunavirDarunavir(TMC114) is an HIV protease inhibitor.IC50 Value: Target: HIV ProteaseDarunavir HIV-1 antiviral structurally is similar to amprenavir and it is second generation HIV-1-protease inhibitor. Darunavir is a drug used to treat HIV infection. It is in the protease inhibitor class. Prezista is an OARAC recommended treatment option for treatment-naive and treatment-experienced adults and adolescents. |
| Name | darunavir |
|---|---|
| Synonym | More Synonyms |
| Description | Darunavir(TMC114) is an HIV protease inhibitor.IC50 Value: Target: HIV ProteaseDarunavir HIV-1 antiviral structurally is similar to amprenavir and it is second generation HIV-1-protease inhibitor. Darunavir is a drug used to treat HIV infection. It is in the protease inhibitor class. Prezista is an OARAC recommended treatment option for treatment-naive and treatment-experienced adults and adolescents. |
|---|---|
| Related Catalog |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 74-76ºC |
| Molecular Formula | C27H37N3O7S |
| Molecular Weight | 547.664 |
| Exact Mass | 547.235229 |
| PSA | 148.80000 |
| LogP | 3.94 |
| Index of Refraction | 1.620 |
| InChIKey | CJBJHOAVZSMMDJ-HEXNFIEUSA-N |
| SMILES | CC(C)CN(CC(O)C(Cc1ccccc1)NC(=O)OC1COC2OCCC12)S(=O)(=O)c1ccc(N)cc1 |
| Storage condition | -20°C Freezer |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
|
Systematic evaluation of commercially available ultra-high performance liquid chromatography columns for drug metabolite profiling: optimization of chromatographic peak capacity.
J. Chromatogr. A. 1374 , 122-33, (2014) The present study investigated the practical use of modern ultra-high performance liquid chromatography (UHPLC) separation techniques for drug metabolite profiling, aiming to develop a widely applicab... |
|
|
The fate of ritonavir in the presence of darunavir.
Int. J. Pharm. 475(1-2) , 214-26, (2014) This study was the first investigation into the potential of a fixed dose combination of ritonavir and darunavir in the form of dispersible powders prepared by spray drying. A common polymer (hydroxyp... |
|
|
Solid self-nanoemulsifying drug delivery system (S-SNEDDS) of darunavir for improved dissolution and oral bioavailability: In vitro and in vivo evaluation.
Eur. J. Pharm. Sci. 74 , 1-10, (2015) The current study was aimed to investigate the potential of solid self-nanoemulsifying drug delivery system (S-SNEDDS) composed of Capmul MCM C8 (oil), Tween 80 (surfactant) and Transcutol P (co-surfa... |
| (3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl [(2S,3R)-4-{[(4-aminophenyl)sulfonyl](isobutyl)amino}-3-hydroxy-1-phenyl-2-butanyl]carbamate |
| Carbamic acid, N-[(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-, (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester |
| Darunavir |
| TMC-114 |
| (3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl [(2S,3R)-4-{[(4-aminophenyl)sulfonyl](isobutyl)amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate |

