fluoromethane-13c
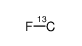
fluoromethane-13c structure
|
Common Name | fluoromethane-13c | ||
|---|---|---|---|---|
| CAS Number | 20666-44-8 | Molecular Weight | 35.02560 | |
| Density | 1.231 (vs air) | Boiling Point | -77.9ºC(lit.) | |
| Molecular Formula | CH3F | Melting Point | -141.8ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS02, GHS04 |
Signal Word | Danger | |
| Name | fluoromethane-13c |
|---|---|
| Synonym | More Synonyms |
| Density | 1.231 (vs air) |
|---|---|
| Boiling Point | -77.9ºC(lit.) |
| Melting Point | -141.8ºC(lit.) |
| Molecular Formula | CH3F |
| Molecular Weight | 35.02560 |
| Exact Mass | 35.02520 |
| LogP | 0.58570 |
| Index of Refraction | 1.212 |
| InChIKey | NBVXSUQYWXRMNV-OUBTZVSYSA-N |
| SMILES | CF |
|
Section1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE Product identifiers Product name: Fluoromethane-13C CAS-No.: 20666-44-8 Section2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP] Flammable gases (Category 1) Gases under pressure (Liquefied gas) Classification according to EU Directives 67/548/EEC or 1999/45/EC Extremely flammable. Label elements Labelling according Regulation (EC) No 1272/2008 [CLP] Pictogram Signal wordDanger Hazard statement(s) Extremely flammable gas. Contains gas under pressure; may explode if heated. Precautionary statement(s) Keep away from heat/sparks/open flames/hot surfaces. - No smoking. P410 + P403Protect from sunlight. Store in a well-ventilated place. Supplemental Hazardnone Statements According to European Directive 67/548/EEC as amended. Hazard symbol(s) R-phrase(s) R12Extremely flammable. S-phrase(s) S16Keep away from sources of ignition - No smoking. S33Take precautionary measures against static discharges. Other hazards - none Section3. COMPOSITION/INFORMATION ON INGREDIENTS Substances Synonyms: Methyl-13Cfluoride Formula: 13CH3F Molecular Weight: 35,03 g/mol Section4. FIRST AID MEASURES Description of first aid measures General advice Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Wash off with soap and plenty of water. Consult a physician. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Most important symptoms and effects, both acute and delayed narcosis, Acts as a simple asphyxiant by displacing air., Dizziness, Disorientation, Headache, excitement, Central nervous system depression, To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. Indication of any immediate medical attention and special treatment needed no data available Section5. FIREFIGHTING MEASURES Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides, Hydrogen fluoride Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information Use water spray to cool unopened containers. Section6. ACCIDENTAL RELEASE MEASURES Personal precautions, protective equipment and emergency procedures Avoid breathing vapors, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Environmental precautions Do not let product enter drains. Methods and materials for containment and cleaning up Clean up promptly by sweeping or vacuum. Reference to other sections For disposal see section 13. Section7. HANDLING AND STORAGE Precautions for safe handling Normal measures for preventive fire protection. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Contents under pressure. Specific end uses no data available Section8. EXPOSURE CONTROLS/PERSONAL PROTECTION Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday. Personal protective equipment Eye/face protection Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Body Protection impervious clothing, The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator with multi-purpose combination (US) or type AXBEK (EN 14387) respirator cartridges as a backup to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Section9. PHYSICAL AND CHEMICAL PROPERTIES Information on basic physical and chemical properties a) AppearanceForm: Liquefied gas Colour: colourless b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingMelting point/range: -141,8 °C - lit. point f) Initial boiling point and -77,9 °C - lit. boiling range g) Flash pointno data available h) Evaporation rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerUpper explosion limit: 10 %(V) flammability orLower explosion limit: 5,0 %(V) explosive limits k) Vapour pressure58,9 hPa at 21,1 °C l) Vapour density1,21 - (Air = 1.0) m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Autoignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available Section10. STABILITY AND REACTIVITY Reactivity no data available Chemical stability no data available Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agentsStrong oxidizing agents, Chemically active metals, Potassium, Aluminum, Magnesium, Zinc Hazardous decomposition products Other decomposition products - no data available Section11. TOXICOLOGICAL INFORMATION Information on toxicological effects Acute toxicity no data available Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitization no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Potential health effects InhalationMay be harmful if inhaled. May cause respiratory tract irritation. IngestionMay be harmful if swallowed. SkinMay be harmful if absorbed through skin. May cause skin irritation. EyesMay cause eye irritation. Signs and Symptoms of Exposure narcosis, Acts as a simple asphyxiant by displacing air., Dizziness, Disorientation, Headache, excitement, Central nervous system depression, To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. Additional Information RTECS: Not available Section12. ECOLOGICAL INFORMATION Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment no data available Other adverse effects no data available Section13. DISPOSAL CONSIDERATIONS Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional waste disposal service to dispose of this material. Contaminated packaging Dispose of as unused product. Section14. TRANSPORT INFORMATION UN number ADR/RID: 2454IMDG: 2454IATA: 2454 UN proper shipping name ADR/RID: METHYL FLUORIDE IMDG: METHYL FLUORIDE IATA:Methyl fluoride Passenger Aircraft: Not permitted for transport Transport hazard class(es) ADR/RID: 2.1IMDG: 2.1IATA: 2.1 Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available Section15. REGULATORY INFORMATION This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006. Safety, health and environmental regulations/legislation specific for the substance or mixture no data available Chemical Safety Assessment SECTION 16 - ADDITIONAL INFORMATION N/A |
| Symbol |


GHS02, GHS04 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H220-H280 |
| Precautionary Statements | P210-P377-P381-P410 + P403 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
| Hazard Codes | F+: Highly flammable; |
| Risk Phrases | R12 |
| Safety Phrases | 16-33 |
| RIDADR | UN 2454 2.1 |
|
Theoretical studies on identity S(N)2 reactions of lithium halide and methyl halide: a microhydration model.
J. Mol. Model. 16(12) , 1931-7, (2010) Reactions of lithium halide (LiX, X = F, Cl, Br and I) and methyl halide (CH₃X, X = F, Cl, Br and I) have been investigated at the B3LYP/6-31G(d) level of theory using the microhydration model. Beginn... |
|
|
A-band methyl halide dissociation via electronic curve crossing as studied by electron energy loss spectroscopy.
J. Chem. Phys. 133(5) , 054304, (2010) Excitation of the A-band low-lying electronic states in the methyl halides, CH(3)I, CH(3)Br, CH(3)Cl, and CH(3)F, has been investigated for the (n-->sigma*) transitions, using electron energy loss spe... |
|
|
Near-thermal reactions of Au(+)(1S,3D) with CH3X (X = F,Cl).
J. Phys. Chem. A 116(3) , 943-51, (2012) Reactions of Au(+)((1)S) and Au(+)((3)D) with CH(3)F and CH(3)Cl have been carried out in a drift cell in He at a pressure of 3.5 Torr at both room temperature and reduced temperatures in order to exp... |
| <13C>-methyl fluoride |
| <13C>-Methylfluorid |
| methyl fluoride-13C |
| MFCD00083967 |