Potassium oxonate
Modify Date: 2025-08-20 12:28:21
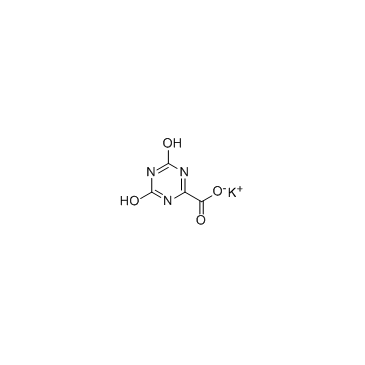
Potassium oxonate structure
|
Common Name | Potassium oxonate | ||
|---|---|---|---|---|
| CAS Number | 2207-75-2 | Molecular Weight | 195.175 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C4H2KN3O4 | Melting Point | 300 °C(lit.) | |
| MSDS | N/A | Flash Point | N/A | |
Use of Potassium oxonateOxonic acid potassium salt is an inhibitor of uricase, oxonic inhibits the phosphorylation of 5-FU to 5-fluorouridine-5'-monophosphate catalyzed by pyrimidine phosphoribosyl-transferase in a different manner from allopurinol in cell-free extracts and intact cells in vitro.IC50 value: Target: On p.o. administration of 5-FU (2 mg/kg) and a potent inhibitor of 5-FU degradation to Yoshida sarcoma-bearing rats, oxonic acid (10 mg/kg) was found to inhibit the formation of 5-fluorouridine-5'-monophosphate from 5-FU and its subsequent incorporation into the RNA fractions of small and large intestine but not of tumor and bone marrow tissues [1]. Oxonic acid diet increased plasma uric acid by 80-90 micromol/l, while blood pressure was elevated only in hyperuricemic 5/6 nephrectomy rats (18 mmHg) [2]. |
| Name | potassium,4,6-dioxo-1H-1,3,5-triazine-2-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | Oxonic acid potassium salt is an inhibitor of uricase, oxonic inhibits the phosphorylation of 5-FU to 5-fluorouridine-5'-monophosphate catalyzed by pyrimidine phosphoribosyl-transferase in a different manner from allopurinol in cell-free extracts and intact cells in vitro.IC50 value: Target: On p.o. administration of 5-FU (2 mg/kg) and a potent inhibitor of 5-FU degradation to Yoshida sarcoma-bearing rats, oxonic acid (10 mg/kg) was found to inhibit the formation of 5-fluorouridine-5'-monophosphate from 5-FU and its subsequent incorporation into the RNA fractions of small and large intestine but not of tumor and bone marrow tissues [1]. Oxonic acid diet increased plasma uric acid by 80-90 micromol/l, while blood pressure was elevated only in hyperuricemic 5/6 nephrectomy rats (18 mmHg) [2]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 300 °C(lit.) |
|---|---|
| Molecular Formula | C4H2KN3O4 |
| Molecular Weight | 195.175 |
| Exact Mass | 194.968231 |
| PSA | 118.74000 |
| InChIKey | IAPCTXZQXAVYNG-UHFFFAOYSA-M |
| SMILES | O=C([O-])c1nc(=O)[nH]c(=O)[nH]1.[K+] |
| Storage condition | Refrigerator, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~80% 
Potassium oxonate CAS#:2207-75-2 |
| Literature: Poje, M.; Sokolic-Maravic, Lea Tetrahedron, 1986 , vol. 42, # 2 p. 747 - 752 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933699090 |
|---|---|
| Summary | 2933699090 other compounds containing an unfused triazine ring (whether or not hydrogenated) in the structure。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:20.0% |
| 1,3,5-Triazine-2-carboxylic acid, 1,4,5,6-tetrahydro-4,6-dioxo-, potassium salt (1:1) |
| Allantoxanic acid |
| monopotassium 1,2,3,4-tetrahydro-2,4-dioxo-1,3,5-triazine-6-carboxylate |
| Potassium Oxonate |
| Oxonic acid potassium salt |
| Oxonic acid |
| Potassium 4,6-Dihydroxy-1,3,5-triazine-2-carboxylate |
| Potassium azaorotate |
| Oxonate |
| MFCD00010565 |
| Potassium Allantoxanate |
| 5-Aza-orotsaeure K-Salz |
| potassium 1,4,5,6-tetrahydro-4,6-dioxo-1,3,5-triazine-2-carboxylate |
| Potassium 4,6-dioxo-1,4,5,6-tetrahydro-1,3,5-triazine-2-carboxylate |
| Allantoxanic Acid Potassium Salt |
| Oxonic acid,potassium salt |
| Oteracil potassium |
| Oxonate,potassium |
| EINECS 218-627-5 |