Cyclothiazide
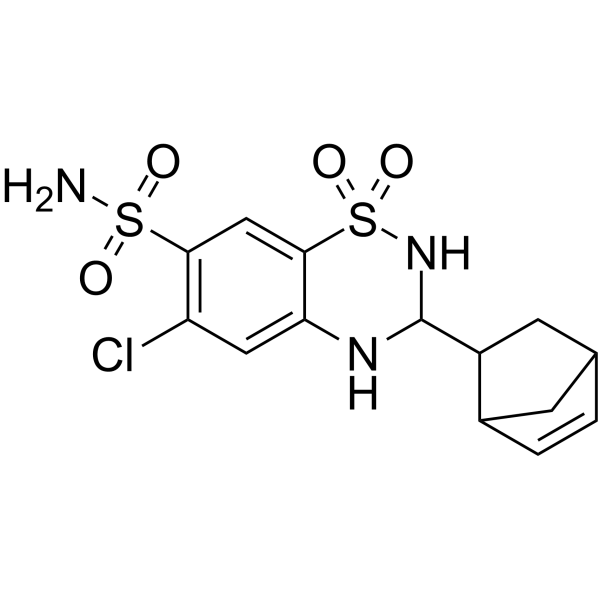
Cyclothiazide structure
|
Common Name | Cyclothiazide | ||
|---|---|---|---|---|
| CAS Number | 2259-96-3 | Molecular Weight | 389.878 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 627.3±65.0 °C at 760 mmHg | |
| Molecular Formula | C14H16ClN3O4S2 | Melting Point | 234ºC | |
| MSDS | Chinese USA | Flash Point | 333.2±34.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CyclothiazideCyclothiazide, a positive allosteric modulator of AMPA receptors, is used frequently to block the desensitization of both native and heterologously expressed AMPA receptors. Cyclothiazide is known to produce a fast inhibition of AMPA receptor desensitization and a much slower potentiation of the AMPA current[1]. |
| Name | cyclothiazide |
|---|---|
| Synonym | More Synonyms |
| Description | Cyclothiazide, a positive allosteric modulator of AMPA receptors, is used frequently to block the desensitization of both native and heterologously expressed AMPA receptors. Cyclothiazide is known to produce a fast inhibition of AMPA receptor desensitization and a much slower potentiation of the AMPA current[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 627.3±65.0 °C at 760 mmHg |
| Melting Point | 234ºC |
| Molecular Formula | C14H16ClN3O4S2 |
| Molecular Weight | 389.878 |
| Flash Point | 333.2±34.3 °C |
| Exact Mass | 389.027069 |
| PSA | 135.12000 |
| LogP | 1.15 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.654 |
| InChIKey | BOCUKUHCLICSIY-UHFFFAOYSA-N |
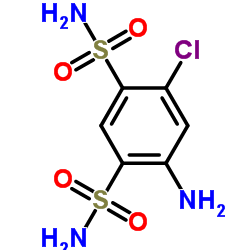
| SMILES | NS(=O)(=O)c1cc2c(cc1Cl)NC(C1CC3C=CC1C3)NS2(=O)=O |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | DK9610000 |
|
~% 
Cyclothiazide CAS#:2259-96-3 |
| Literature: Journal of Organic Chemistry, , vol. 26, p. 2814 - 2818 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Nicotinic receptors modulate the function of presynaptic AMPA receptors on glutamatergic nerve terminals in the trigeminal caudal nucleus.
Neurochem. Int. 90 , 166-72, (2015) In this study, we demonstrate the existence on trigeminal caudal nucleus (TCN) glutamatergic terminals of α4β2 nicotinic receptors (nAChRs) capable of enhancing the terminals' spontaneous release of [... |
|
|
AMPA receptor desensitization is the determinant of AMPA receptor mediated excitotoxicity in purified retinal ganglion cells.
Exp. Eye Res. 132 , 136-50, (2015) The ionotropic glutamate receptors (iGLuR) have been hypothesized to play a role in neuronal pathogenesis by mediating excitotoxic death. Previous studies on iGluR in the retina have focused on two br... |
|
|
Molecular mechanisms contributing to TARP regulation of channel conductance and polyamine block of calcium-permeable AMPA receptors.
J. Neurosci. 34(35) , 11673-83, (2014) Many properties of fast synaptic transmission in the brain are influenced by transmembrane AMPAR regulatory proteins (TARPs) that modulate the pharmacology and gating of AMPA-type glutamate receptors ... |
| cyclothiazidum [INN_la] |
| Cyclothiazide |
| 3-(5-bicyclo[2.2.1]hept-2-enyl)-6-chloro-1,1-dioxo-3,4-dihydro-2H-1λ<sup>6</sup>,2,4-benzothiadiazine-7-sulfonamide |
| UNII:P71U09G5BW |
| 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 3-bicyclo[2.2.1]hept-5-en-2-yl-6-chloro-3,4-dihydro-, 1,1-dioxide |
| 3-(Bicyclo[2.2.1]hept-5-en-2-yl)-6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide |
| 6-Chloro-3,4-dihydro-3-(2-norbornen-5-yl)-2H-1,2-4-benzothiadiazine-7-sulfonamide 1,1-dioxide |
| MFCD00210192 |