Triallate

Triallate structure
|
Common Name | Triallate | ||
|---|---|---|---|---|
| CAS Number | 2303-17-5 | Molecular Weight | 304.664 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 322.1±52.0 °C at 760 mmHg | |
| Molecular Formula | C10H16Cl3NOS | Melting Point | 29-30°C | |
| MSDS | Chinese USA | Flash Point | 148.6±30.7 °C | |
| Symbol |



GHS07, GHS08, GHS09 |
Signal Word | Warning | |
Use of TriallateTriallate is a selective preemergence herbicide for the control of wild oats in barley, spring wheat, Durum wheat, winter wheat, and sugar beets. Triallate inhibits fatty acid elongation and surface lipid (wax) biosynthesis[1][2]. |
| Name | tri-allate |
|---|---|
| Synonym | More Synonyms |
| Description | Triallate is a selective preemergence herbicide for the control of wild oats in barley, spring wheat, Durum wheat, winter wheat, and sugar beets. Triallate inhibits fatty acid elongation and surface lipid (wax) biosynthesis[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 322.1±52.0 °C at 760 mmHg |
| Melting Point | 29-30°C |
| Molecular Formula | C10H16Cl3NOS |
| Molecular Weight | 304.664 |
| Flash Point | 148.6±30.7 °C |
| Exact Mass | 303.001831 |
| PSA | 45.61000 |
| LogP | 6.18 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.532 |
| Water Solubility | 4.0 g/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS07, GHS08, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H317-H373-H410 |
| Precautionary Statements | P273-P280-P501 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful;N: Dangerous for the environment; |
| Risk Phrases | R22;R43;R48/22;R50/53 |
| Safety Phrases | S24-S37-S60-S61-S36-S26-S16 |
| RIDADR | UN 3077 |
| RTECS | EZ8575000 |
| Packaging Group | I; II; III |
| Hazard Class | 6.1 |
| HS Code | 2930200013 |
|
~% 
Triallate CAS#:2303-17-5 |
| Literature: Synthesis, , # 8 p. 622 - 623 |
| HS Code | 2930200013 |
|---|---|
| Summary | 2930200013 (z)-s-(2,3-dichloroallyl) diisopropylcarbamothioate。supervision conditions:s(import or export registration certificate for pesticides)。VAT:17.0%。tax rebate rate:9.0%。MFN tarrif:6.5%。general tariff:30.0% |
|
Carry-over of dinitramine, triallate, and trifluralin to the following spring in soils treated at different times during the fall.
Bull. Environ. Contam. Toxicol. 29(4) , 483-6, (1982)
|
|
|
Volatilization modeling of two herbicides from soil in a wind tunnel experiment under varying humidity conditions.
Environ. Sci. Technol. 46(22) , 12527-33, (2012) Volatilization of pesticides from the bare soil surface is drastically reduced when the soil is under dry conditions (i.e., water content lower than the permanent wilting point). This effect is caused... |
|
|
Body mass index and bromoxynil exposure in a sample of rural residents during spring herbicide application.
J. Toxicol. Environ. Health A 67(17) , 1321-52, (2004) Bromoxynil (3,5-dibromo-4-hydroxybenzonitrile), a phenolic herbicide, is widely used in production of cereals and other crops. Little is known, however, about bromoxynil exposure in humans. Results of... |
| S-(2,3,3-trichloro-2-propen-1-yl) N,N-bis(1-methylethyl)carbamothioate |
| s-2,3,3-trichloroallyl diisopropylthiocarbamate |
| S-(2,3,3-trichloroprop-2-en-1-yl) dipropan-2-ylcarbamothioate |
| Triamyl |
| Diisopropylthiocarbamate de S-(2,3,3-trichloro-2-propèn-1-yle) |
| N,N-diisopropylthiocarbamic acid 2,3,3-trichloroallyl ester |
| S-(2,3,3-trichloroprop-2-en-1-yl) di(propan-2-yl)carbamothioate |
| S-(2,3,3-trichloroprop-2-enyl) N,N-di(propan-2-yl)carbamothioate |
| S-(2,3,3-Trichloroprop-2-en-1-yl) diisopropylcarbamothioate |
| Triallate |
| Bis(1-methylethyl)carbamothioic acid S-(2,3,3-trichloro-2-propenyl) ester |
| S-2,3,3-trichloroallyl diisopropyl(thiocarbamate) |
| MFCD00078730 |
| S-(2,3,3-Trichloro-2-propenyl) bis(1-methylethyl)carbamothioate (9CI) |
| S-(2,3,3-Trichloroallyl) diisopropylthiocarbamate (8CI) |
| Caswell No. 870A |
| Far-Go |
| EINECS 218-962-7 |
| S-(2,3,3-Trichlorprop-2-en-1-yl)-diisopropylthiocarbamat |
| Carbamothioic acid, N,N-bis(1-methylethyl)-, S-(2,3,3-trichloro-2-propen-1-yl) ester |
| S-(2,3,3-Trichlor-2-propen-1-yl)-diisopropylthiocarbamat |
| S-(2,3,3-trichloroprop-2-en-1-yl) diisopropylthiocarbamate |
| S-(2,3,3-Trichloro-2-propen-1-yl) diisopropylcarbamothioate |
| Carbamothioic acid, bis(1-methylethyl)-, S-(2,3,3-trichloro-2-propenyl) ester |
| Triallat |
| Avadex BW |
| S-(2,3,3-trichloroprop-2-en-1-yl) bis(1-methylethyl)thiocarbamate |
| S-2,3,3-trichloroallyl diisopropyl thiocarbamate |
| Dipthal |

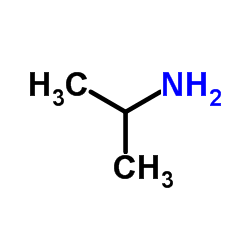 CAS#:75-31-0
CAS#:75-31-0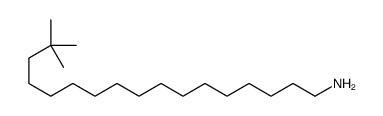 CAS#:71133-38-5
CAS#:71133-38-5
