Decitabine
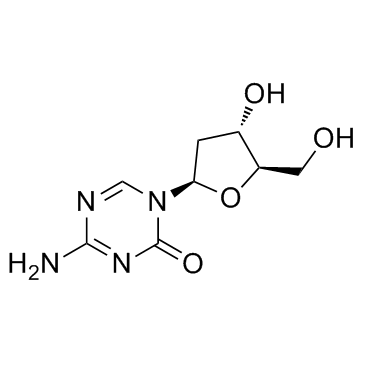
Decitabine structure
|
Common Name | Decitabine | ||
|---|---|---|---|---|
| CAS Number | 2353-33-5 | Molecular Weight | 228.205 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 485.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C8H12N4O4 | Melting Point | ~200 °C (dec.) | |
| MSDS | Chinese USA | Flash Point | 247.6±31.5 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of DecitabineDecitabine (NSC 127716) is a DNA methyltransferase inhibitor commonly used to treat myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). |
| Name | 5-aza-2'-deoxycytidine |
|---|---|
| Synonym | More Synonyms |
| Description | Decitabine (NSC 127716) is a DNA methyltransferase inhibitor commonly used to treat myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). |
|---|---|
| Related Catalog | |
| Target |
DNMT1 DNMT3A DNMT3B |
| In Vitro | Decitabine treatment significantly inhibits cell growth of SNU719, NCC24 and KATOIII 96 hours after exposure to decitabine. Decitabine induces G2/M arrest and apoptosis in EBVaGC, inhibits invasion ability, and up-regulates E-cadherin expression for EBVaGC[1]. Tri-acetylation on the H4 N-terminal tail (H4K8acK12acK16ac) is reduced after DAC treatment in MDS-L sensitive cells[2]. Decitabine up-regulates DCTPP1 and dUTPase expression in HeLa cells[3]. |
| In Vivo | Decitabine (1.0 mg/kg, p.o.) in combination with tetrahydrouridine (THU) causes severe toxicity occurs in females, and results in an increased sensitivity to decitabine toxicity correlating with decitabine plasma levels[4]. |
| Kinase Assay | The pyrophosphohydrolase activity of DCTPP1 is determined using a continuous spectrophotometric assay. In a standard reaction (1 mL final volum), 10-250 μM of the nucleotide substrate is incubated in reaction buffer (20 mM MgCl2, 100 mM KCl, 0.75 mg/mL BSA and 4 mM DTT) with concentrations ranging from 0.1-1 μM of DCTTP1. All reactions are carried out at 25°C. |
| Cell Assay | Cell viability is analyzed by cell count and MTS assay. SNU719, NCC24 and KATOIII are seeded at a density of 5×104 cells/mL and cultured with only RPMI-1640 supplemented with FBS for 24 hours. After 24 hours of incubation, cells are treated in the presence or absence of Decitabine (DAC) for 120 hours. Cells for cell counts are trypsinized and counted at 0, 24, 48, 72, 96 and 120 hours after DAC treatment. Viable cells are determined by trypan blue exclusion. For MTS assay, cells (SNU719 and NCC24: 1x104/well, KATOIII: 1x103/well) are eeded onto 96-well dishes. After seeding, MTS is added into the well at the indicated period. After incubation for 1 hour, the absorbance is measured at 490 nm. |
| Animal Admin | Mice are assigned to four dose groups and a vehicle control group. Animals are gavaged with Decitabine (DAC) or its vehicle 1 hour ± 5 minutes after administration of tetrahydrouridine (THU) or its vehicle at a dose volume of 10 mL/kg. The DAC doses are selected based on the range finding study in which the mice tolerated six oral doses (2x/week) of 0.1, 0.2 and 0.4 mg/kg DAC in combination with a fixed dose of 167 mg/kg THU. A fixed THU dose (500 mg/m2) and the optimal timing between THU and DAC administration (60 min) are selected. Conversion of milligrams per body surface area dose in mice into milligrams per kilogram body weight dose estimation is based on Michaelis constant (Km) values for mice. In brief, the mouse dose in milligrams per body surface area (500 mg/m2) is divided by the Km of 3 to convert the dose to milligrams per kilogram body weight (167 mg/kg). The working body weight range of mice in the guideline is 11-34 gram; the body weight range of mice used in this study is 24-38 gram. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 485.8±55.0 °C at 760 mmHg |
| Melting Point | ~200 °C (dec.) |
| Molecular Formula | C8H12N4O4 |
| Molecular Weight | 228.205 |
| Flash Point | 247.6±31.5 °C |
| Exact Mass | 228.085861 |
| PSA | 123.49000 |
| LogP | -1.93 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.780 |
| Stability | Stable. May be light or air sensitive. Incompatible with strong oxidizing agents. |
| Water Solubility | acetic acid/water (1:1): 50 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H319-H335-H341-H360 |
| Precautionary Statements | P201-P261-P281-P305 + P351 + P338-P308 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | XZ3012000 |
|
~% 
Decitabine CAS#:2353-33-5 |
| Literature: EP2371825 A1, ; Page/Page column 9 ; |
|
~% 
Decitabine CAS#:2353-33-5 |
| Literature: EP2048151 A1, ; Page/Page column 3 ; |
|
~% 
Decitabine CAS#:2353-33-5 |
| Literature: US2010/36112 A1, ; Page/Page column 7-8 ; |
|
~98% 
Decitabine CAS#:2353-33-5 |
| Literature: Cilag AG Patent: EP2050757 A1, 2009 ; Location in patent: Page/Page column 4 ; |
|
~% 
Decitabine CAS#:2353-33-5 |
| Literature: US2010/249394 A1, ; Page/Page column 6 ; |
|
~% 
Decitabine CAS#:2353-33-5 |
| Literature: WO2010/129211 A2, ; Page/Page column 28 ; |
|
~% 
Decitabine CAS#:2353-33-5 |
| Literature: US2006/14949 A1, ; Page/Page column 6; 15 ; |
|
~% 
Decitabine CAS#:2353-33-5 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 70, # 11 p. 1228 - 1232 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |
|
The high mobility group A2 protein epigenetically silences the Cdh1 gene during epithelial-to-mesenchymal transition.
Nucleic Acids Res. 43(1) , 162-78, (2015) The loss of the tumour suppressor E-cadherin (Cdh1) is a key event during tumourigenesis and epithelial-mesenchymal transition (EMT). Transforming growth factor-β (TGFβ) triggers EMT by inducing the e... |
|
|
Epigenetic variation in the Egfr gene generates quantitative variation in a complex trait in ants.
Nat. Commun. 6 , 6513, (2015) Complex quantitative traits, like size and behaviour, are a pervasive feature of natural populations. Quantitative trait variation is the product of both genetic and environmental factors, yet little ... |
|
|
Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis.
J. Clin. Invest. 124(7) , 3187-99, (2014) In atherosclerosis, plaques preferentially develop in arterial regions of disturbed blood flow (d-flow), which alters endothelial gene expression and function. Here, we determined that d-flow regulate... |
| 4-Amino-1-(2-deoxy-β-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one |
| s-Triazin-2(1H)-one, 4-amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)- |
| MFCD00043011 |
| 5-AZA-CDR |
| DAC |
| 5-AZA-DC |
| Decitabine |
| 5-DEOXY-2'-AZACYTIDINE |
| EINECS 219-089-4 |
| 1,3,5-Triazin-2(1H)-one, 4-amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)- |
| 4-Amino-1-(2-deoxy-b-D-erythro-pentofuranosyl)-1,3,5-triazin-2(1H)-one |
| 5-Aza-1-(2-deoxy-β-D-ribofuranosyl)cytosine |
| 4-Amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)-1,3,5-triazin-2(1H)-one |
| 2'-deoxy-5-azacytidine |
| 5-Aza-2'-deoxycytidine |
| Dacogen |
| 2-Desoxy-5-azacytidine |
| 5-azadeoxycytidine |
| 5-Aza-2′-deoxycytidine |
| 5-Aza-2‘-deoxycytidine |
| 4-Amino-1-(2-deoxy-β-D-erythro-pentofuranosyl)-1,3,5-triazin-2(1H)-on |
| 4-Amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-1,3,5-triazin-2(1H)-one |
| 4-Amino-1-(2-deoxy-b-D-erythro-pentofuranosyl)-s-triazin-2(1H)-one |

![N-(Trimethylsilyl)-4-[(trimethylsilyl)oxy]-1,3,5-triazin-2-amine structure](https://image.chemsrc.com/caspic/089/52523-35-0.png)
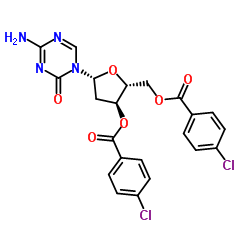
![4-amino-1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-alpha-D-ribofuranosyl-1,3,5-triazin-2(1H)-one structure](https://image.chemsrc.com/caspic/116/1140891-02-6.png)
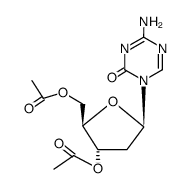
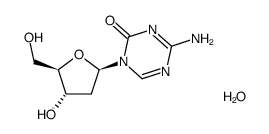
![N-[N-[[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]carbamoyl]carbamimidoyl]formamide structure](https://image.chemsrc.com/caspic/067/69304-64-9.png)
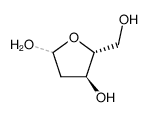 CAS#:452-51-7
CAS#:452-51-7
