6-Hydroxyindole
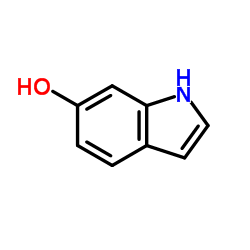
6-Hydroxyindole structure
|
Common Name | 6-Hydroxyindole | ||
|---|---|---|---|---|
| CAS Number | 2380-86-1 | Molecular Weight | 133.15 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 343.2±15.0 °C at 760 mmHg | |
| Molecular Formula | C8H7NO | Melting Point | 124-130 °C | |
| MSDS | Chinese USA | Flash Point | 161.4±20.4 °C | |
| Symbol |



GHS05, GHS07, GHS09 |
Signal Word | Danger | |
Use of 6-Hydroxyindole6-Hydroxyindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 1H-indol-6-ol |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Hydroxyindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 343.2±15.0 °C at 760 mmHg |
| Melting Point | 124-130 °C |
| Molecular Formula | C8H7NO |
| Molecular Weight | 133.15 |
| Flash Point | 161.4±20.4 °C |
| Exact Mass | 133.052765 |
| PSA | 36.02000 |
| LogP | 1.41 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.739 |
| InChIKey | XAWPKHNOFIWWNZ-UHFFFAOYSA-N |
| SMILES | Oc1ccc2cc[nH]c2c1 |
| Storage condition | Keep Cold |
| Symbol |



GHS05, GHS07, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H317-H318-H411 |
| Precautionary Statements | P273-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R22;R41;R43;R51/53 |
| Safety Phrases | S37/39-S26-S61-S24-S2 |
| RIDADR | UN 3077 |
| Hazard Class | 9.0 |
| Precursor 8 | |
|---|---|
| DownStream 4 | |
|
Determination of selected synthetic cannabinoids and their metabolites by micellar electrokinetic chromatography--mass spectrometry employing perfluoroheptanoic acid-based micellar phase.
Talanta 150 , 568-76, (2016) Perfluoroheptanoic acid was employed as a volatile micellar phase in background electrolyte for micellar electrokinetic chromatography-tandem mass spectrometry separation and determination of 15 selec... |
|
|
Inhibitory effect of hydroxyindoles and their analogues on human melanoma tyrosinase.
Z. Naturforsch., C, J. Biosci. 65 , 49-54, (2010) A recent study showed that N-acylserotonin derivatives have strong inhibitory activity against tyrosinase. To clarify the role of the 5-hydroxy group in the indole ring, 2-, 4-, 5-, 6-, and 7-hydroxyi... |
|
|
Albumin stimulates the activity of the human UDP-glucuronosyltransferases 1A7, 1A8, 1A10, 2A1 and 2B15, but the effects are enzyme and substrate dependent.
PLoS ONE 8(1) , e54767, (2013) Human UDP-glucuronosyltransferases (UGTs) are important enzymes in metabolic elimination of endo- and xenobiotics. It was recently shown that addition of fatty acid free bovine serum albumin (BSA) sig... |
| indolol |
| 6-hydroxy-1h-indole |
| 1H-Indol-6-ol |
| 6-Hydroxyindole |
| EINECS 417-020-4 |
| indol-6-ol |
| 6-monohydroxyindole |
| 6-hydroxy-indole |
| 6-indolol |
| MFCD00152101 |
![1-[2-(2-nitro-4-phenylmethoxyphenyl)ethenyl]pyrrolidine Structure](https://image.chemsrc.com/caspic/324/153805-86-8.png) CAS#:153805-86-8
CAS#:153805-86-8 CAS#:15903-94-3
CAS#:15903-94-3 CAS#:3189-13-7
CAS#:3189-13-7 CAS#:842117-36-6
CAS#:842117-36-6![[1-(2,2-dimethylpropanoyl)indol-6-yl] 2-chloroacetate Structure](https://image.chemsrc.com/caspic/236/160252-47-1.png) CAS#:160252-47-1
CAS#:160252-47-1![Ethenamine, N,N-dimethyl-2-[2-nitro-4-(phenylmethoxy)phenyl]-, (E) Structure](https://image.chemsrc.com/caspic/435/99474-13-2.png) CAS#:99474-13-2
CAS#:99474-13-2 CAS#:24239-67-6
CAS#:24239-67-6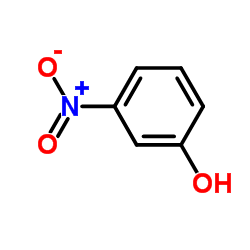 CAS#:554-84-7
CAS#:554-84-7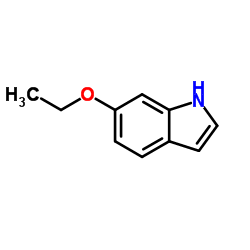 CAS#:37865-86-4
CAS#:37865-86-4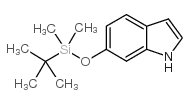 CAS#:106792-41-0
CAS#:106792-41-0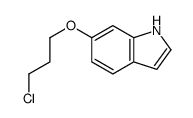 CAS#:70260-96-7
CAS#:70260-96-7
