Mycophenolic acid
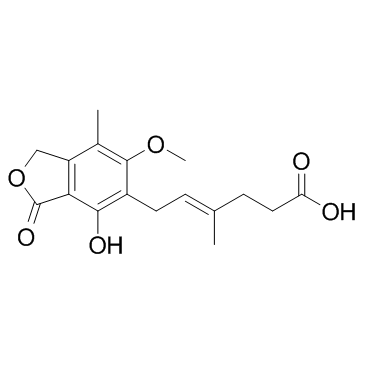
Mycophenolic acid structure
|
Common Name | Mycophenolic acid | ||
|---|---|---|---|---|
| CAS Number | 24280-93-1 | Molecular Weight | 320.337 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 611.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C17H20O6 | Melting Point | 141°C | |
| MSDS | Chinese USA | Flash Point | 225.8±25.0 °C | |
| Symbol |



GHS07, GHS08, GHS09 |
Signal Word | Danger | |
Use of Mycophenolic acidMycophenolic acid is an an immunosuppresant drug and has potent anti-proliferative activity.Target: OthersMycophenolic acid(MPA) is an immunosuppressant drug used to prevent rejection in organ transplantation. It inhibits an enzyme needed for the growth of T cells and B cells. MPA did not block the initial phase of viral translation but did interfere with viral protein synthesis in the amplification phase. Quantitative RT-PCR demonstrated that MPA prevented the accumulation of viral positive- and negative-strand RNA as the infection proceeded. MPA inhibits flavivirus infection by preventing synthesis and accumulation of viral RNA [1]. The effects of mycophenolic acid (MPA) on DEN replication in monkey kidney (LLC-MK2) cells were examined. MPA (IC(50)=0.4+/-0.3 microM) inhibited DEN2 replication. Quantitative real-time RT-PCR of viral RNA and plaque assays of virions from DEN2-infected and MPA (10 microM) -treated cells showed a fivefold increase in defective viral RNA production by cells treated with each drug. suggesting that one mode of antiviral action of MPA is by inhibition of inosine monophosphate dehydrogenase and thereby depletion of the intracellular GTP pool [2]. |
| Name | Mycophenolic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Mycophenolic acid is an an immunosuppresant drug and has potent anti-proliferative activity.Target: OthersMycophenolic acid(MPA) is an immunosuppressant drug used to prevent rejection in organ transplantation. It inhibits an enzyme needed for the growth of T cells and B cells. MPA did not block the initial phase of viral translation but did interfere with viral protein synthesis in the amplification phase. Quantitative RT-PCR demonstrated that MPA prevented the accumulation of viral positive- and negative-strand RNA as the infection proceeded. MPA inhibits flavivirus infection by preventing synthesis and accumulation of viral RNA [1]. The effects of mycophenolic acid (MPA) on DEN replication in monkey kidney (LLC-MK2) cells were examined. MPA (IC(50)=0.4+/-0.3 microM) inhibited DEN2 replication. Quantitative real-time RT-PCR of viral RNA and plaque assays of virions from DEN2-infected and MPA (10 microM) -treated cells showed a fivefold increase in defective viral RNA production by cells treated with each drug. suggesting that one mode of antiviral action of MPA is by inhibition of inosine monophosphate dehydrogenase and thereby depletion of the intracellular GTP pool [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 611.6±55.0 °C at 760 mmHg |
| Melting Point | 141°C |
| Molecular Formula | C17H20O6 |
| Molecular Weight | 320.337 |
| Flash Point | 225.8±25.0 °C |
| Exact Mass | 320.125977 |
| PSA | 93.06000 |
| LogP | 2.92 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.585 |
| InChIKey | HPNSFSBZBAHARI-RUDMXATFSA-N |
| SMILES | COc1c(C)c2c(c(O)c1CC=C(C)CCC(=O)O)C(=O)OC2 |
| Storage condition | 2-8°C |
| Water Solubility | methanol: 50 mg/mL, clear, colorless to faintly yellow |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS07, GHS08, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H341-H360D-H372-H410 |
| Precautionary Statements | P201-P273-P281-P308 + P313-P501 |
| Target Organs | Immune system |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S53-S45 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| RTECS | MP8050000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2932209090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
A nicotinic receptor-mediated anti-inflammatory effect of the flavonoid rhamnetin in BV2 microglia.
Fitoterapia 98 , 11-21, (2014) The alpha7 nicotinic acetylcholine receptor (nAChR) is a potential target in neuroinflammation. Screening a plant extract library identified Solidago nemoralis as containing methyl-quercetin derivativ... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
| Mycophenolic acid |
| melbex |
| 6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methylhex-4-enoic acid |
| EINECS 246-119-3 |
| micofenolicoacido |
| 4-Hexenoic acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl- |
| 6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methyl-4-hexenoic acid |
| Micophenolic acid |
| Mycophenolinsure |
| Mycophenolic |
| mycophenolate |
| MFCD00036814 |
| 6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid |
| Mycophenolate Mofetil Impurity 6 |
 CAS#:31858-66-9
CAS#:31858-66-9 CAS#:35334-60-2
CAS#:35334-60-2 CAS#:100045-79-2
CAS#:100045-79-2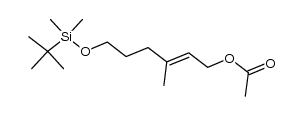 CAS#:100045-80-5
CAS#:100045-80-5![(E)-3-methyl-6-[(tert-butyldimethylsilyl)oxy]-2-hexen-1-ol Structure](https://image.chemsrc.com/caspic/271/100045-81-6.png) CAS#:100045-81-6
CAS#:100045-81-6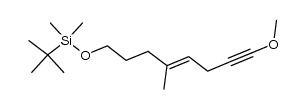 CAS#:100045-82-7
CAS#:100045-82-7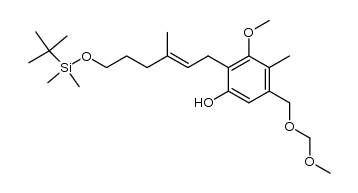 CAS#:100045-85-0
CAS#:100045-85-0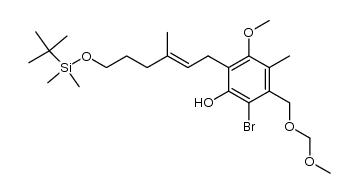 CAS#:100045-86-1
CAS#:100045-86-1 CAS#:60435-90-7
CAS#:60435-90-7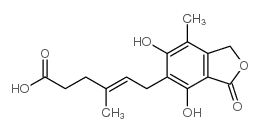 CAS#:31858-65-8
CAS#:31858-65-8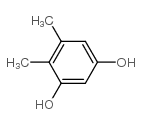 CAS#:527-55-9
CAS#:527-55-9![7-hydroxy-5-methoxy-4-methyl-6-[2-(2-methyl-5-oxooxolan-2-yl)ethyl]-3H-2-benzofuran-1-one structure](https://image.chemsrc.com/caspic/422/26675-76-3.png) CAS#:26675-76-3
CAS#:26675-76-3![3-(5-methoxy-2,6-dimethyl-9-oxo-4,7-dihydro-3H-furo[3,4-h]chromen-2-yl)propanoic acid structure](https://image.chemsrc.com/caspic/017/24243-38-7.png) CAS#:24243-38-7
CAS#:24243-38-7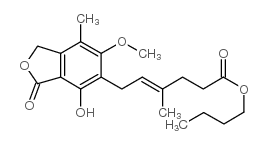 CAS#:27004-95-1
CAS#:27004-95-1 CAS#:115007-34-6
CAS#:115007-34-6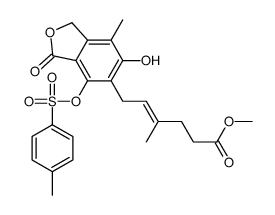 CAS#:171808-04-1
CAS#:171808-04-1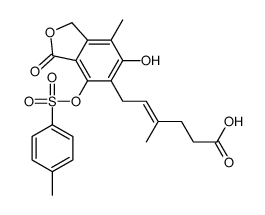 CAS#:171808-03-0
CAS#:171808-03-0
