Bufexamac
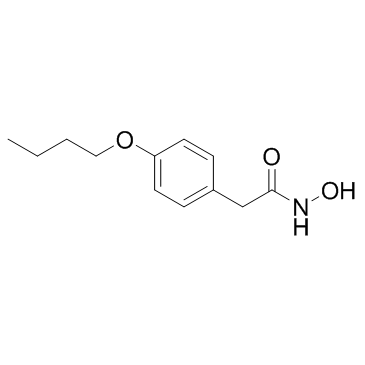
Bufexamac structure
|
Common Name | Bufexamac | ||
|---|---|---|---|---|
| CAS Number | 2438-72-4 | Molecular Weight | 223.268 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C12H17NO3 | Melting Point | 161 - 162ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of BufexamacBufexamac is a class IIB histone deacetylases (HDAC6 and HDAC10) inhibitor used as an anti-inflammatory agent. |
| Name | bufexamac |
|---|---|
| Synonym | More Synonyms |
| Description | Bufexamac is a class IIB histone deacetylases (HDAC6 and HDAC10) inhibitor used as an anti-inflammatory agent. |
|---|---|
| Related Catalog | |
| Target |
HDAC6:10.7 μM (Kd app) HDAC10:12.3 μM (Kd app) HDAC8:235 μM (Kd app) HDAC3:341 μM (Kd app) |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Melting Point | 161 - 162ºC |
| Molecular Formula | C12H17NO3 |
| Molecular Weight | 223.268 |
| Exact Mass | 223.120850 |
| PSA | 58.56000 |
| LogP | 1.70 |
| Index of Refraction | 1.530 |
| InChIKey | MXJWRABVEGLYDG-UHFFFAOYSA-N |
| SMILES | CCCCOc1ccc(CC(=O)NO)cc1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | AK8280000 |
| HS Code | 2924299090 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Erythema-multiforme-like, urticarial papular and plaque eruptions from bufexamac: report of 4 cases.
Contact Dermatitis 31(2) , 97-101, (1994) 4 patients developed an erythema-multiforme-like reaction following acute contact dermatitis from 2 different bufexamac-containing topical preparations. Histologically, the lesions did not show change... |
|
|
[A severe epicutaneous test reaction to the bufexamac in a hemorrhoidal therapeutic preparation].
Dtsch. Med. Wochenschr. 124(40) , 1168-70, (1999) A 49-year-old woman presented with acute perianal vesicular/bullous contact dermatitis. Other areas were over the trunk, face, neck and wrists. She reported occasional application of an ointment (Mast... |
|
|
[A common and insidious side-effect: allergic contact dermatitis caused by bufexamac used in the treatment of dermatitis. Results from the Information Network of Departments of Dermatology (IDVK)].
Dtsch. Med. Wochenschr. 130(50) , 2881-6, (2005) Bufexamac is a non-steroidal, anti-inflammatory drug used in the topical treatment of atopic dermatitis, stasis dermatitis and perianal eczema. The substance is known to cause severe allergic contact ... |
| Feximac |
| Bufexamaco |
| Flogicid |
| Bufexamacum |
| Bufexamic acid |
| MFCD00078936 |
| 2-(4-Butoxyphenyl)-N-hydroxyacetamide |
| 4-butoxy-N-hydroxybenzeneacetamide |
| Droxaryl |
| 2-(4-butoxyphenyl)acetohydroxamic acid |
| 2-(p-Butoxyphenyl)acetohydroxamic acid |
| Benzeneacetamide, 4-butoxy-N-hydroxy- |
| 2-(4-butoxyphenyl)acethydroxamic acid |
| EINECS 219-451-1 |
| Bufexamac |
| Malipuran |
| Parfenac |
 CAS#:4547-57-3
CAS#:4547-57-3 CAS#:7803-49-8
CAS#:7803-49-8