Z-D-Phe-OH
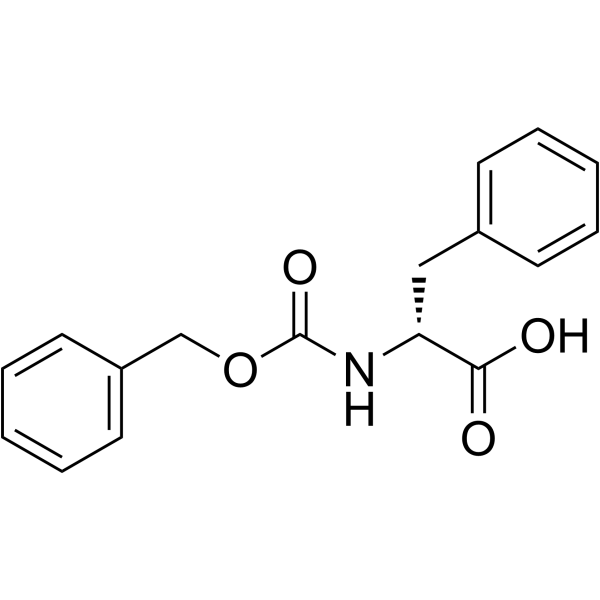
Z-D-Phe-OH structure
|
Common Name | Z-D-Phe-OH | ||
|---|---|---|---|---|
| CAS Number | 2448-45-5 | Molecular Weight | 299.321 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 511.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C17H17NO4 | Melting Point | 80-82ºC | |
| MSDS | Chinese USA | Flash Point | 263.1±30.1 °C | |
Use of Z-D-Phe-OH((Benzyloxy)carbonyl)-D-phenylalanine is a phenylalanine derivative[1]. |
| Name | (2R)-3-phenyl-2-(phenylmethoxycarbonylamino)propanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | ((Benzyloxy)carbonyl)-D-phenylalanine is a phenylalanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 511.5±50.0 °C at 760 mmHg |
| Melting Point | 80-82ºC |
| Molecular Formula | C17H17NO4 |
| Molecular Weight | 299.321 |
| Flash Point | 263.1±30.1 °C |
| Exact Mass | 299.115753 |
| PSA | 75.63000 |
| LogP | 3.58 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.591 |
| InChIKey | RRONHWAVOYADJL-OAHLLOKOSA-N |
| SMILES | O=C(NC(Cc1ccccc1)C(=O)O)OCc1ccccc1 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Effects of metalloendoproteinase inhibitors on secretion and intracellular free calcium in bovine adrenal chromaffin cells.
Biochim. Biophys. Acta 889(1) , 1-5, (1986) The possible role of metalloendoproteinase in stimulus-secretion coupling in adrenal chromaffin cells was examined using the metalloendoproteinase inhibitors 1,10-phenanthroline and carbobenzoxy-Gly-P... |
|
|
Mechanism of the binding of Z-L-tryptophan and Z-L-phenylalanine to thermolysin and stromelysin-1 in aqueous solutions.
Biochim. Biophys. Acta 1824(2) , 303-10, (2012) The chemical shift of the carboxylate carbon of Z-tryptophan is increased from 179.85 to 182.82 ppm and 182.87 ppm on binding to thermolysin and stromelysin-1 respectively. The chemical shift of Z-phe... |
|
|
Modification of a zinc proteinase from Bacillus mesentericus strain 76 by diethylpyrocarbonate.
Int. J. Pept. Protein Res. 37(4) , 325-30, (1991) Diethylpyrocarbonate (DEPC) inactivated the neutral zinc proteinase from Bacillus mesentericus strain 76/Bacillus subtilis (MCP 76) by ethoxycarbonylation completely. Exposure of the enzyme to DEPC to... |
| N-Cbz-D-phenylalanine Z-D-phenylalanine |
| EINECS 258-154-1 |
| MFCD00063151 |
| L-Phenylalanine, N-[(phenylmethoxy)carbonyl]- |
| Cbz-D-Phenylalanine |
| Z-D-phenylalanine |
| Cbz-protected D-phenylalanine |
| Phenylalanine, N-[(phenylmethoxy)carbonyl]- |
| N-CBZ-L-phenylalanine |
| N-[(Benzyloxy)carbonyl]phenylalanine |
| (2R)-2-{[(benzyloxy)carbonyl]amino}-3-phenylpropanoic acid |
| N-Cbz-D-Phenylalanine |
| Cbz-D-Phe-OH |
| N-carbobenzyloxy-dl-phenylalanine |
| N-CARBOBENZYLOXY-D-PHENYLALANINE |
| Z-D-Phe-OH |
| (2S)-2-{[(benzyloxy)carbonyl]amino}-3-phenylpropanoic acid |
| N-Carbobenzoxy-D-phenylalanine |
| Benzyloxycarbonyl-D-phenylalanine |
 CAS#:673-06-3
CAS#:673-06-3 CAS#:501-53-1
CAS#:501-53-1 CAS#:2578-85-0
CAS#:2578-85-0 CAS#:565234-20-0
CAS#:565234-20-0 CAS#:247259-40-1
CAS#:247259-40-1![benzyl N-[(2S)-3-bromo-2-hydroxypropyl]carbamate Structure](https://image.chemsrc.com/caspic/158/247050-08-4.png) CAS#:247050-08-4
CAS#:247050-08-4 CAS#:565234-16-4
CAS#:565234-16-4 CAS#:565234-15-3
CAS#:565234-15-3 CAS#:64-17-5
CAS#:64-17-5 CAS#:3588-57-6
CAS#:3588-57-6 CAS#:17350-84-4
CAS#:17350-84-4 CAS#:23239-35-2
CAS#:23239-35-2![Benzyl [(2R)-1-oxo-3-phenyl-2-propanyl]carbamate structure](https://image.chemsrc.com/caspic/297/63219-70-5.png) CAS#:63219-70-5
CAS#:63219-70-5 CAS#:58607-69-5
CAS#:58607-69-5 CAS#:58970-76-6
CAS#:58970-76-6 CAS#:58917-85-4
CAS#:58917-85-4 CAS#:3397-36-2
CAS#:3397-36-2 CAS#:153223-21-3
CAS#:153223-21-3
