Iperoxo
Modify Date: 2025-09-19 18:07:43
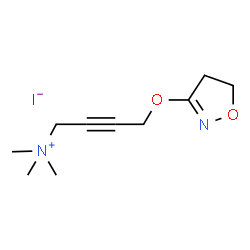
Iperoxo structure
|
Common Name | Iperoxo | ||
|---|---|---|---|---|
| CAS Number | 247079-84-1 | Molecular Weight | 324.159 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C10H17IN2O2 | Melting Point | 211.5 - 212.5 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of IperoxoAn extremely potent muscarinic receptor agonist with EC50 of 2.12 nM and 8.47 nM for M2 and M4, respectively; significantly exceeds ACh in Gi /Gs signalling competence CHO-hM2 cells. |
| Name | 4-(4,5-Dihydro-1,2-oxazol-3-yloxy)-N,N,N-trimethyl-2-butyn-1-aminium iodide |
|---|---|
| Synonym | More Synonyms |
| Description | An extremely potent muscarinic receptor agonist with EC50 of 2.12 nM and 8.47 nM for M2 and M4, respectively; significantly exceeds ACh in Gi /Gs signalling competence CHO-hM2 cells. |
|---|---|
| References | References 1. Schrage R, et al. Br J Pharmacol. 2013 May;169(2):357-70. 2. Kruse AC, et al. Nature. 2013 Dec 5;504(7478):101-6. 3. Croy CH, et al. Mol Pharmacol. 2014 Jul;86(1):106-15. View Related Products by Target mAChR |
| Melting Point | 211.5 - 212.5 °C |
|---|---|
| Molecular Formula | C10H17IN2O2 |
| Molecular Weight | 324.159 |
| Exact Mass | 324.033478 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| RIDADR | NONH for all modes of transport |
|
Bis(ammonio)alkane-type agonists of muscarinic acetylcholine receptors: synthesis, in vitro functional characterization, and in vivo evaluation of their analgesic activity.
Eur. J. Med. Chem. 75 , 222-32, (2014) In this study, we synthesized and tested in vitro and in vivo two groups of bis(ammonio)alkane-type compounds, 6a-9a and 6b-9b, which incorporate the orthosteric muscarinic agonist iperoxo into a mole... |
| 4-(4,5-Dihydro-1,2-oxazol-3-yloxy)-N,N,N-trimethyl-2-butyn-1-aminium iodide |
| 2-Butyn-1-aminium, 4-[(4,5-dihydro-3-isoxazolyl)oxy]-N,N,N-trimethyl-, iodide (1:1) |
| MFCD26793886 |