Povidone Iodine
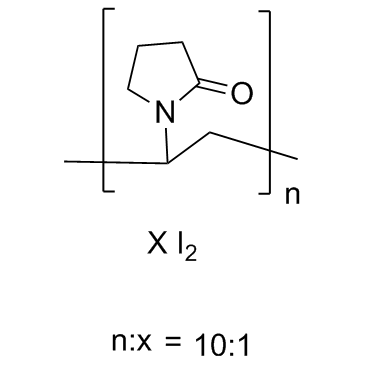
Povidone Iodine structure
|
Common Name | Povidone Iodine | ||
|---|---|---|---|---|
| CAS Number | 25655-41-8 | Molecular Weight | 364.951 | |
| Density | N/A | Boiling Point | 217.6ºC at 760 mmHg | |
| Molecular Formula | C6H9I2NO | Melting Point | 300ºC | |
| MSDS | Chinese USA | Flash Point | 93.9ºC | |
Use of Povidone IodinePovidone iodine displays excellent antibacterial activity which can against MRSA and MSSA strains with MICs of 31.25 mg/L and 7.82 mg/L, respectively. |
| Name | 1-ethenylpyrrolidin-2-one,molecular iodine |
|---|---|
| Synonym | More Synonyms |
| Description | Povidone iodine displays excellent antibacterial activity which can against MRSA and MSSA strains with MICs of 31.25 mg/L and 7.82 mg/L, respectively. |
|---|---|
| Related Catalog | |
| In Vitro | MIC values of Povidone iodine (PVP-I) are 31.25 mg/L and 7.82 mg/L, respectively. Treatment of the cells with Povidone iodine (PVP-I) at a dilution of 1:32 causes a sharp reduction in cell viability by 90-95% on all testing cell lines[1]. |
| In Vivo | The Dp+Povidone iodine (PVP-I) group has the second highest average score from day 13 to the end of the experimental period. The Dp+Povidone iodine and Dp+Et-OH groups also show a significantly increase in eosinophil count compare with the control group (p<0.05 and p<0.001, respectively). However, the eosinophil count does not significantly differ among the Dp+Povidone iodine (PVP-I), Dp+CHG, and Dp+vehicle groups[2]. |
| Cell Assay | Each bacterial isolate is washed twice with phosphate-buffered saline (PBS, pH 7.2), centrifuged for 10 min at 1932xg at 20°C, and suspended in 3 mL of nutrient broth, adjusted to a turbidity equivalent of 0.5 McFarland standard. The bacterial suspension is diluted 1:100 with MHB to a final inoculum of 106 colony-forming units (cfu)/mL. For each bacterial strain, two rows of a 96-well microtitre plate are filled with the final bacterial inoculum (50 μL per well) and 50 μL of each serial dilution of ILαD. The procedure is repeated for the Povidone iodine (PVP-I) serial dilutions[1]. |
| Animal Admin | The mice are divided into 6 groups as follows: 1) saline+vehicle (control group), 2) Dp+vehicle, 3) Dp+BZK, 4) Dp+Povidone iodine (PVP-I), 5) Dp+Et-OH, and 6) Dp+CHG. Animals in the experimental groups are exposed to the allergen through the subcutaneous injection of 5 µg of Dp dissolving in 10 µL of saline in the ventral side of the right ear 2 to 3 days a week (a total of 8 times) under anesthesia with 4% halothane. Animals in the control group are not sensitized, receiving a subcutaneous injection of 10 µL of saline in the ventral side of the right ear. Animals receive an application of antiseptic agent are exposed to the allergen and treated with 0.2% (w/v) benzalkonium chloride (Dp+BZK), 10% (w/v) povidone-iodine (Dp+PVP-I), 80% (v/v) ethanol (Dp+Et-OH) or 0.5% (v/v) chlorhexidine gluconate (Dp+CHG). These agents are applied a total of 15 times during the experimental period. The BZK, Povidone iodine (PVP-I), Et-OH, and CHG are dissolved in 25 µL of injection water and applied gently to the dorsal side of the right ear using a micropipette with a fine plastic tip. The animals in the Dp+vehicle and control groups receive 25 µL of injection water. All animals are sacrificed on the last day of the experiment (day 18)[2]. |
| References |
| Boiling Point | 217.6ºC at 760 mmHg |
|---|---|
| Melting Point | 300ºC |
| Molecular Formula | C6H9I2NO |
| Molecular Weight | 364.951 |
| Flash Point | 93.9ºC |
| Exact Mass | 364.877319 |
| PSA | 20.31000 |
| LogP | 2.46160 |
| Vapour Pressure | 0.132mmHg at 25°C |
| InChIKey | CPKVUHPKYQGHMW-UHFFFAOYSA-N |
| SMILES | C=CN1CCCC1=O.II |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| RTECS | TR1579600 |
| HS Code | 3808940010 |
| HS Code | 3808940010 |
|---|
|
Diplacone and mimulone ameliorate dextran sulfate sodium-induced colitis in rats.
Fitoterapia 101 , 201-7, (2015) Diplacone (1) and mimulone (2), two geranylated flavanones, have previously shown anti-inflammatory and antiradical activity in vitro. The present study aimed to evaluate their activity in vivo on a m... |
|
|
Povidone-iodine and hydrogen peroxide mixture soaked gauze pack: a novel hemostatic technique.
J. Oral Maxillofac. Surg. 71(11) , 1833.e1-1833.e3, (2013) Persistent oozing of blood is a common occurrence in maxillofacial surgery, and occasionally it hampers visibility and delays or even prevents continuation of the procedure. This report describes a no... |
|
|
Effect of low-concentration povidone iodine on postoperative complications after third molar surgery: a pilot split-mouth study.
J. Oral Maxillofac. Surg. 73(1) , 18-21, (2015) Povidone iodine is used primarily as an antiseptic to decrease surgical site infection. Its hemostatic and antiedematous properties in oral surgery also have been investigated recently.A randomized co... |
| Isobetadyne |
| Povidone-iodine |
| Disphex |
| Polyvinylpyrrolidone-Iodine Complex |
| PVP-I |
| Poly(vinylpyrrolidone)-Iodine complex |
| MFCD00084483 |
| Povidone (iodinated) |
| EINECS 215-034-3 |
| Poly(vinylpyrrolidone)–Iodine complex |
| Isodine |
| Bridine |
| PVP iodine |
| Betadine |
| Povidone Iodine |

