CDN-A
Modify Date: 2025-11-27 23:25:44
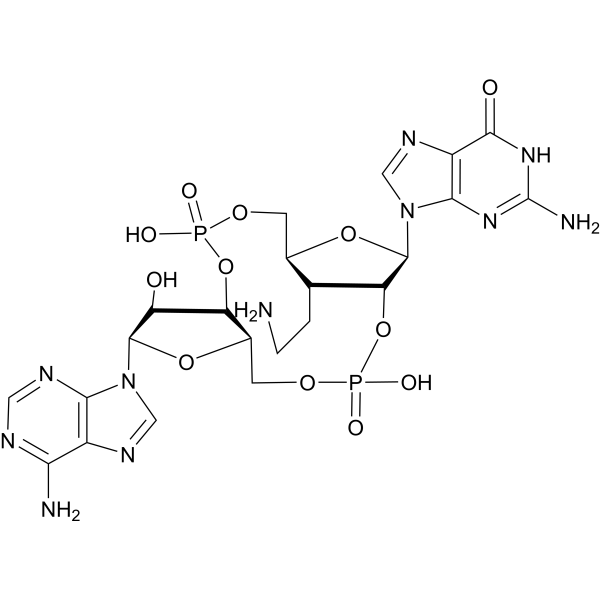
CDN-A structure
|
Common Name | CDN-A | ||
|---|---|---|---|---|
| CAS Number | 2586047-09-6 | Molecular Weight | 701.48 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H29N11O12P2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of CDN-ACDN-A is a cyclic di-nucleotide, it can be used to synthesis antibody-drug conjugate (ADC). Cyclic di-nucleotides are potent stimulators of innate and adaptive immune responses. In humans, cyclic di-nucleotide, which are either produced endogenously in response to foreign DNA or by invading bacterial pathogens, trigger the innate immune system by activating the expression of interferon genes[1][2][3]. |
| Name | CDN-A |
|---|
| Description | CDN-A is a cyclic di-nucleotide, it can be used to synthesis antibody-drug conjugate (ADC). Cyclic di-nucleotides are potent stimulators of innate and adaptive immune responses. In humans, cyclic di-nucleotide, which are either produced endogenously in response to foreign DNA or by invading bacterial pathogens, trigger the innate immune system by activating the expression of interferon genes[1][2][3]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C22H29N11O12P2 |
|---|---|
| Molecular Weight | 701.48 |