Phthalimidine, 2-(2,6-dioxopiperiden-3-yl).
Modify Date: 2025-09-01 15:17:30
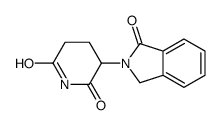
Phthalimidine, 2-(2,6-dioxopiperiden-3-yl). structure
|
Common Name | Phthalimidine, 2-(2,6-dioxopiperiden-3-yl). | ||
|---|---|---|---|---|
| CAS Number | 26581-81-7 | Molecular Weight | 244.24600 | |
| Density | 1.393g/cm3 | Boiling Point | 534.3ºC at 760mmHg | |
| Molecular Formula | C13H12N2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 276.9ºC | |
Use of Phthalimidine, 2-(2,6-dioxopiperiden-3-yl).2-(2,6-Dioxopiperidin-3-yl)phthalimidine (EM-12), a teratogenic Thalidomide analogue, is more active than Thalidomide and is much more stable for hydrolysis. 2-(2,6-Dioxopiperidin-3-yl)phthalimidine enhances 1,2-dimethylhydrazine-induction of rat colon adenocarcinomas[1][2]. |
| Name | 3-(1-Oxo-1,3-dihydro-2H-isoindol-2-yl)-2,6-piperidinedione |
|---|---|
| Synonym | More Synonyms |
| Description | 2-(2,6-Dioxopiperidin-3-yl)phthalimidine (EM-12), a teratogenic Thalidomide analogue, is more active than Thalidomide and is much more stable for hydrolysis. 2-(2,6-Dioxopiperidin-3-yl)phthalimidine enhances 1,2-dimethylhydrazine-induction of rat colon adenocarcinomas[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Young male Sprague-Dawley rats in 3 groups are fed a basal diet supplemented with 0.10 wt. % each of thalidomide and its imide-analog of much higher teratogenicity, 2-(2,6-Dioxopiperidin-3-yl)phthalimidine. Following an induction period of 17 days on the diets, all animals are injected subcutaneously with 1,2-dimethylhydrazine at 20 mg/kg for a total of 20 weekly doses and killed on week 18 after the 20th injection. 2-(2,6-Dioxopiperidin-3-yl)phthalimidine-fed group elicits statistically significant increases both in the total and ascending colon-based adenocarcinomas as compared with the control findings, in keeping with its greater teratogenicity and embryotoxicity[2]. |
| References |
| Density | 1.393g/cm3 |
|---|---|
| Boiling Point | 534.3ºC at 760mmHg |
| Molecular Formula | C13H12N2O3 |
| Molecular Weight | 244.24600 |
| Flash Point | 276.9ºC |
| Exact Mass | 244.08500 |
| PSA | 69.97000 |
| LogP | 0.66140 |
| InChIKey | WENKGSGGXGQHSH-UHFFFAOYSA-N |
| SMILES | O=C1CCC(N2Cc3ccccc3C2=O)C(=O)N1 |
| 2-(1-Oxoisoindolin-2-yl)-glutarimid |
| 2-(2,6-dioxopiperdin-3-yl)-1-oxoisoindoline |
| 3-[1,3-dihydro-1-oxo-2H-isoindol-2-yl]-2,6-dioxopiperidine |
| (+/-)-Hydroxythalidomide |
| 5-Hydroxythalidomide |
| 2-(2,6-dioxo-3-piperidinyl)-5-hydroxy-1H-isoindole-1,3(2H)-dione |
| 1H-Isoindole-1,3(2H)-dione,2-(2,6-dioxo-3-piperidinyl)-5-hydroxy |
| 2-(2,6-dioxo-piperidin-3-yl)-5-hydroxy-isoindole-1,3-dione |
| 3-(1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione |