Aminooxyacetic acid hemihydrochloride
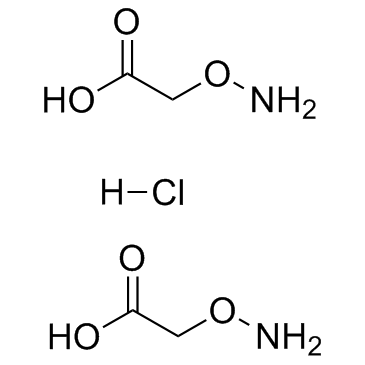
Aminooxyacetic acid hemihydrochloride structure
|
Common Name | Aminooxyacetic acid hemihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 2921-14-4 | Molecular Weight | 109.30 | |
| Density | N/A | Boiling Point | 326.7ºC at 760 mmHg | |
| Molecular Formula | NH2OCH2COOH·0.5HCl | Melting Point | 156 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 151.4ºC | |
Use of Aminooxyacetic acid hemihydrochlorideAminooxyacetic acid hemihydrochloride is a malate-aspartate shuttle (MAS) inhibitor which also inhibits the GABA degradating enzyme GABA-T. |
| Name | Carboxymethoxylamine hemihydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Aminooxyacetic acid hemihydrochloride is a malate-aspartate shuttle (MAS) inhibitor which also inhibits the GABA degradating enzyme GABA-T. |
|---|---|
| Related Catalog | |
| Target |
MAS[1], GABA-T[2] |
| In Vitro | Aminooxyacetic acid hemihydrochloride (AOAA) dose-dependently decreases the survival of C6 glioma cells. Aminooxyacetic acid hemihydrochloride treatment produces a significant increase in the percentage of the cells arrested in the stage of G0/G1, as well as a significant decrease in the percentage of the cells at S phase and G2/M phase. Aminooxyacetic acid hemihydrochloride treatment leads to an obvious decrease in the number of the cells in the phase of cell division. Aminooxyacetic acid hemihydrochloride significantly increases the percentage of the cells in both early-stage apoptosis and necrosis. Treatment of the cells with 1 mM or 5 mM Aminooxyacetic acid hemihydrochloride leads to decreased levels of aging of the cells[1]. Glutamine-dependent cell lines show greater inhibition of cell growth by Aminooxyacetic acid hemihydrochloride (AOA) compare with cells that are less glutamine dependent[3]. |
| In Vivo | The accumulation of GABA in cerebellum and whole brain is initially very rapid, being significantly increased already 5 min after the injection of Aminooxyacetic acid hemihydrochloride (AOAA). The rapid initial accumulation becomes gradually slower and maximal levels (400 to 600 % of the control levels) are reached 2 to 6 h after Aminooxyacetic acid hemihydrochloride. Still 24 h after Aminooxyacetic acid hemihydrochloride the GABA levels are elevated by about 250%. From 2 to 6 h after Aminooxyacetic acid hemihydrochloride the convulsions are completely blocked. Twenty four hours after Aminooxyacetic acid hemihydrochloride the convulsions are almost identical to the controls[2]. |
| Kinase Assay | Enzyme activity of aspartate transaminase is measured by a colorimetric assay assessing formation of pyruvate from oxaloacetate, a product of GOT1/2 (also called AST1/2) activity, as described previously. In brief, cells grown in 6-well plates are collected after 6, 24, or 48 hours of Aminooxyacetic acid hemihydrochloride (AOA) treatment and washed with cold PBS, lysed, and supernatant used for analysis[3]. |
| Cell Assay | Breast cancer cell lines are used in this study. Cells are plated in 96-well plates at 1,500 to 5,000 cells per well in 100 μL media. New medium with varying concentration of Aminooxyacetic acid hemihydrochloride (AOA) is added after 12 hours. The assay is performed after 48 hours[3]. |
| Animal Admin | Female albino rats (150 to 200 g) bred at our department are used. Aminooxyacetic acid hemihydrochloride (AOAA) is injected into a tail vein (2 mL/kg body weight). For this purpose the rat is placed in a plastic tube and the tail warmed in water 42°C[2]. |
| References |
| Boiling Point | 326.7ºC at 760 mmHg |
|---|---|
| Melting Point | 156 °C (dec.)(lit.) |
| Molecular Formula | NH2OCH2COOH·0.5HCl |
| Molecular Weight | 109.30 |
| Flash Point | 151.4ºC |
| PSA | 145.10000 |
| LogP | 0.12520 |
| Vapour Pressure | 4.24E-05mmHg at 25°C |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | UN 1193 3/PG 2 |
| WGK Germany | 3 |
| RTECS | AF3150000 |
| Packaging Group | II |
| Hazard Class | 3 |
|
~% 
Aminooxyacetic ... CAS#:2921-14-4 |
| Literature: Anker; Clarke Org.Synth.Coll.Vol.III<1955>172 Full Text Show Details Lott Journal of the American Chemical Society, 1948 , vol. 70, p. 1972 Full Text View citing articles Show Details Borek; Clarke Journal of the American Chemical Society, 1936 , vol. 58, p. 2020 |
| Precursor 1 | |
|---|---|
| DownStream 10 | |
|
Synthesis and in vitro antibacterial properties of 2-(3-bromo-5-isoxazolylideneamino-oxy)acetamido-beta-lactam derivatives.
Il Farmaco 46 , 887, (1991) The synthesis of new 2-(3-bromo-5-isoxazolylideneamino-oxy)acetic acids and their condensation derivatives with suitable beta-lactam nuclei is reported. Their antibacterial properties have been tested... |
|
|
Tetrahedron Lett. 34 , 4347, (1993)
|
| O-Carboxymethylhydroxylamine hemihydrochloride |
| (Aminooxy)acetic acid hydrochloride (2:1) |
| 2-aminooxyacetic acid |
| (carboxymethoxy)amine hemihydrochloride |
| Acetic acid, 2-(aminooxy)-, hydrochloride (2:1) |
| Carboxymethoxylamine-D2 |
| Aminyloxy-acetic Acid Hemihydrochlorid |
| 2-AMINOOXY-ACETICACIHYDROCHLORIDE |
| aminooxyaceticacidhemichloride |
| AMinooxyacetic acid Hydrochloride |
| O-(carboxymethyl) hydroxylamine hemichloride |
| 2-aminooxy-aceticacihydrochloride(2:1) |
| Bis((aminooxy)acetic) acid hydrochloride |
| MFCD00012955 |
| Aminooxyacetic acid hemihydrochloride |
| 2-(Aminooxy)acetic acid hydrochloride(2:1) |
| (Aminooxy)acetic Acid Hemihydrochloride |
| (aminooxy)-aceticacihydrochloride(2:1) |
| CarboxyMethoxylaMine HeMihydrochloride |
| O-(Carboxymethyl)hydroxylamine hemihydrochloride |
| Carboxymethoxyamine hemihydrochloride |
| EINECS 220-862-3 |
| (carbomethoxy)amine hemihydrochloride |
![[[(1-methylethylidene)amino]oxy]acetic acid structure](https://image.chemsrc.com/caspic/241/5382-89-8.png)
 CAS#:35048-47-6
CAS#:35048-47-6 CAS#:42989-85-5
CAS#:42989-85-5 CAS#:37654-41-4
CAS#:37654-41-4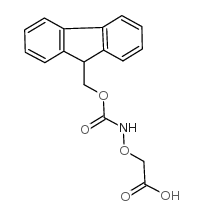 CAS#:123106-21-8
CAS#:123106-21-8![[[(α-Methylbenzylidene)amino]oxy]acetic acid structure](https://image.chemsrc.com/caspic/427/1205-09-0.png) CAS#:1205-09-0
CAS#:1205-09-0![2-[bis(2-chloroethyl)aminooxy]acetic acid structure](https://image.chemsrc.com/caspic/106/838893-18-8.png) CAS#:838893-18-8
CAS#:838893-18-8![2-[(E)-1-(4-ethyl-3-oxo-1,4-benzoxazin-6-yl)ethylideneamino]oxyacetic acid structure](https://image.chemsrc.com/caspic/422/91119-64-1.png) CAS#:91119-64-1
CAS#:91119-64-1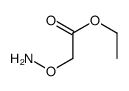 CAS#:5740-47-6
CAS#:5740-47-6![2-[(6-oxocyclohexa-2,4-dien-1-ylidene)methylamino]oxyacetic acid structure](https://image.chemsrc.com/caspic/283/62713-03-5.png) CAS#:62713-03-5
CAS#:62713-03-5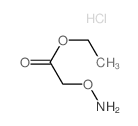 CAS#:3919-73-1
CAS#:3919-73-1
