Nicotinic acid mononucleotide
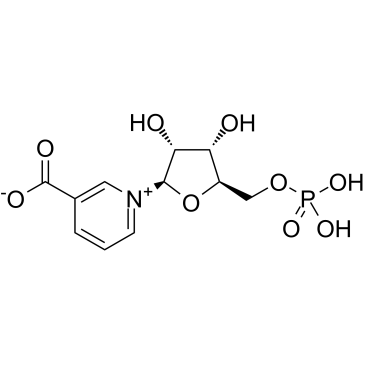
Nicotinic acid mononucleotide structure
|
Common Name | Nicotinic acid mononucleotide | ||
|---|---|---|---|---|
| CAS Number | 321-02-8 | Molecular Weight | 336.21200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H14NO9P | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Nicotinic acid mononucleotideNicotinic acid mononucleotide (NAMN) is formed from nicotinic acid (NA) via the nicotinic acid phosphoribosyltransferase in the biosynthesis of NAD+. Nicotinate mononucleotide is a substrate for nicotinamide mononucleotide/Nicotinic acid mononucleotide adenylyltransferase[1][2]. |
| Name | 3-Carboxy-1-(5-O-phosphono-β-D-ribofuranosyl)pyridinium |
|---|---|
| Synonym | More Synonyms |
| Description | Nicotinic acid mononucleotide (NAMN) is formed from nicotinic acid (NA) via the nicotinic acid phosphoribosyltransferase in the biosynthesis of NAD+. Nicotinate mononucleotide is a substrate for nicotinamide mononucleotide/Nicotinic acid mononucleotide adenylyltransferase[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C11H14NO9P |
|---|---|
| Molecular Weight | 336.21200 |
| Exact Mass | 336.04800 |
| PSA | 167.44000 |
| InChIKey | JOUIQRNQJGXQDC-ZYUZMQFOSA-L |
| SMILES | O=C([O-])c1ccc[n+](C2OC(COP(=O)([O-])[O-])C(O)C2O)c1 |
| Storage condition | 20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
ArsAB, a novel enzyme from Sporomusa ovata activates phenolic bases for adenosylcobamide biosynthesis.
Mol. Microbiol. 81(4) , 952-67, (2011) In the homoacetogenic bacterium Sporomusa ovata, phenol and p-cresol are converted into α-ribotides, which are incorporated into biologically active cobamides (Cbas) whose lower ligand bases do not fo... |
|
|
Dissecting cobamide diversity through structural and functional analyses of the base-activating CobT enzyme of Salmonella enterica.
Biochim. Biophys. Acta 1840(1) , 464-75, (2014) Cobamide diversity arises from the nature of the nucleotide base. Nicotinate mononucleotide (NaMN):base phosphoribosyltransferases (CobT) synthesize α-linked riboside monophosphates from diverse nucle... |
|
|
Characterization of human nicotinate phosphoribosyltransferase: Kinetic studies, structure prediction and functional analysis by site-directed mutagenesis.
Biochimie 94(2) , 300-9, (2012) Nicotinate phosphoribosyltransferase (NaPRT, EC 2.4.2.11) catalyzes the conversion of nicotinate (Na) to nicotinate mononucleotide, the first reaction of the Preiss-Handler pathway for the biosynthesi... |
| 3-Carboxy-1-methylpyridiniumiodid |
| Pyridinium,3-carboxy-1-methyl-,iodide |
| Trigonelline hydriodide |
| trigonelline hydroiodide |
| nicotinic acid mononucleotide |
| Nicotinsaeure-iodmethylat |
| Nicotinsaeure-mononucleotid |
| nicotinic acid methiodide |
| nicotinate adenine mononucleotide |
| 3-Carboxy-1-methyl-pyridinium,Jodid |
| 3-carboxy-1-methyl-pyridinium,iodide |
 CAS#:89-00-9
CAS#:89-00-9 CAS#:97-55-2
CAS#:97-55-2