1-Fluoronaphthalene
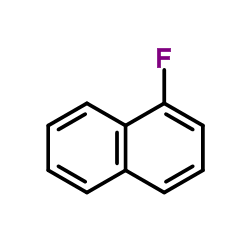
1-Fluoronaphthalene structure
|
Common Name | 1-Fluoronaphthalene | ||
|---|---|---|---|---|
| CAS Number | 321-38-0 | Molecular Weight | 146.161 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 215.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H7F | Melting Point | −13 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 65.6±0.0 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of 1-Fluoronaphthalene1-Fluoronaphthalene is an organofluorine compound derived from naphthalene derivatives and fluorinated aromatics. 1-Fluoronaphthalene can be used to synthesize LY248686, a potent inhibitor of serotonin and noradrenaline uptake[1]. |
| Name | Fluoronaphthalene |
|---|---|
| Synonym | More Synonyms |
| Description | 1-Fluoronaphthalene is an organofluorine compound derived from naphthalene derivatives and fluorinated aromatics. 1-Fluoronaphthalene can be used to synthesize LY248686, a potent inhibitor of serotonin and noradrenaline uptake[1]. |
|---|---|
| Related Catalog | |
| In Vitro | 1-Fluoronaphthalene (3 mg, 24 h) can be oxidized by C.elegans ATCC 36112 to form trans-3,4-dihydroxy-3,4-dihydro-1-fluoronaphthalene and trans-5,6-dihydroxy-5,6-dihydro-1-fluoronaphthalene and to form 1-fluoro-8-hydroxy-5-tetralone, 5-hydroxy-1-fluoronaphthalene and 4-hydroxy-1-fluoronaphthalene as well as glucosides, sulfates and glucuronide conjugates of these phenols[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 215.0±0.0 °C at 760 mmHg |
| Melting Point | −13 °C(lit.) |
| Molecular Formula | C10H7F |
| Molecular Weight | 146.161 |
| Flash Point | 65.6±0.0 °C |
| Exact Mass | 146.053177 |
| LogP | 3.50 |
| Vapour Pressure | 0.2±0.4 mmHg at 25°C |
| Index of Refraction | 1.606 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S36/37-S24/25-S23-S53 |
| RIDADR | UN 2810 |
| WGK Germany | 3 |
| RTECS | QJ7100000 |
| HS Code | 2903999090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2903999090 |
|---|---|
| Summary | 2903999090 halogenated derivatives of aromatic hydrocarbons VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Synthesis of highly oxygenated dinaphthyl ethers via SNAr reactions promoted by Barton's base.
Org. Lett. 5(7) , 1155-8, (2003) [reaction: see text] Electron-rich dinaphthyl ethers were synthesized by S(N)Ar reactions between naphthols and activated fluoronaphthalenes. 2-tert-Butyl-1,1,3,3-tetramethylguanidine (Barton's base) ... |
|
|
Effects of a fluoro substituent on the fungal metabolism of 1-fluoronaphthalene.
Appl. Environ. Microbiol. 48(2) , 294-300, (1984) The metabolism of 1-fluoronaphthalene by Cunninghamella elegans ATCC 36112 was studied. The metabolites were isolated by reverse-phase high-pressure liquid chromatography and characterized by the appl... |
|
|
A one-pot access to 6-substituted phenanthridines from fluoroarenes and nitriles via 1,2-arynes.
Org. Lett. 4(16) , 2687-90, (2002) [reaction: see text] A one-pot, t-BuLi-induced synthesis of 6-substituted phenanthridines from fluoroarenes and nitriles via 1,2-arynes is reported. Aryl- and hetaryl nitriles, cyanamides, and trimeth... |
| 1-FLUORONAPTHALENE |
| α-Fluoronaphthalene |
| 1-fluoromaphthalene |
| 1-fluoro-naphthalen |
| Naphthalene, 1-fluoro- |
| EINECS 206-287-0 |
| 1-Fluornaphthalen |
| 4-fluoro-l-naphthalene |
| I-Fluoronaphthalene |
| 1-Flouronaphthalene |
| I-Fluornaphthalin |
| L66J BF |
| MFCD00003873 |
| 1-Fluoronaphthalene |
| 1-Fluornaftalen |
| 1-FLUORO NAPHTHALENE |
 CAS#:99747-74-7
CAS#:99747-74-7 CAS#:17938-06-6
CAS#:17938-06-6 CAS#:13922-41-3
CAS#:13922-41-3 CAS#:90-13-1
CAS#:90-13-1 CAS#:3759-61-3
CAS#:3759-61-3 CAS#:90-11-9
CAS#:90-11-9 CAS#:642-28-4
CAS#:642-28-4 CAS#:91-20-3
CAS#:91-20-3 CAS#:972-09-8
CAS#:972-09-8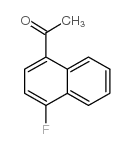 CAS#:316-68-7
CAS#:316-68-7 CAS#:605-62-9
CAS#:605-62-9 CAS#:341-41-3
CAS#:341-41-3 CAS#:317-79-3
CAS#:317-79-3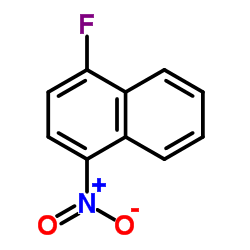 CAS#:341-92-4
CAS#:341-92-4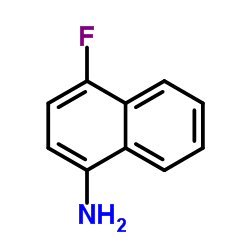 CAS#:438-32-4
CAS#:438-32-4 CAS#:143901-96-6
CAS#:143901-96-6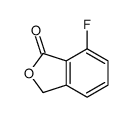 CAS#:2211-82-7
CAS#:2211-82-7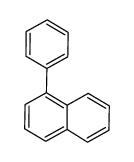 CAS#:605-02-7
CAS#:605-02-7 CAS#:612-94-2
CAS#:612-94-2
