Methyl Piperidine-2-carboxylate
Modify Date: 2025-08-20 14:36:03

Methyl Piperidine-2-carboxylate structure
|
Common Name | Methyl Piperidine-2-carboxylate | ||
|---|---|---|---|---|
| CAS Number | 32559-18-5 | Molecular Weight | 179.645 | |
| Density | N/A | Boiling Point | 235.3ºC at 760 mmHg | |
| Molecular Formula | C7H14ClNO2 | Melting Point | 205ºC (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 96.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | methyl piperidine-2-carboxylate,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 235.3ºC at 760 mmHg |
|---|---|
| Melting Point | 205ºC (dec.)(lit.) |
| Molecular Formula | C7H14ClNO2 |
| Molecular Weight | 179.645 |
| Flash Point | 96.1ºC |
| Exact Mass | 179.071304 |
| PSA | 38.33000 |
| LogP | 1.43230 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933399090 |
| Precursor 3 | |
|---|---|
| DownStream 6 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Aldehyde-based racemization in the dynamic kinetic resolution of N-heterocyclic a-amino esters using Candida Antarctica lipase A. Liljeblad A, et al.
Tetrahedron 60(3) , 671-677, (2004)
|
|
|
Enantioselective lipase-catalyzed reactions of methyl pipecolinate: transesterification and N-acylation. Liljeblad, A, et al.
Tetrahedron Lett. 43(13) , 2471-2474, (2002)
|
|
|
The α-effect in cyclic secondary amines: new scaffolds for iminium ion accelerated transformations. Brazier JB, et al.
Tetrahedron 65(48) , 9961-9966, (2009)
|
| MFCD00192316 |
| piperidine-2-carboxylic acid methyl ester hydrochloride |
| 2-Piperidinecarboxylic acid, methyl ester, hydrochloride (1:1) |
| methyl piperidine-2-carboxylate hydrochloride |
| Methyl 2-piperidinecarboxylate hydrochloride (1:1) |
| L-pipecolic acid methyl ester hydrochloride |
| Methyl pipecolinate hydrochloride |
| DL-pipecolic acid methyl ester hydrochloride |
| Methyl 2-piperidinecarboxylate hydrochloride |
| (S)-pipecolic acid methyl ester hydrochloride |
| 2-piperidine-carboxylic acid methyl ester hydrochloride |
| Methy pipecolinate HCl |
| Methyl Piperidine-2-carboxylate |
 CAS#:67-56-1
CAS#:67-56-1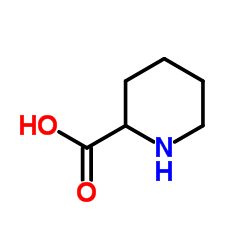 CAS#:4043-87-2
CAS#:4043-87-2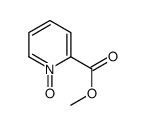 CAS#:38195-81-2
CAS#:38195-81-2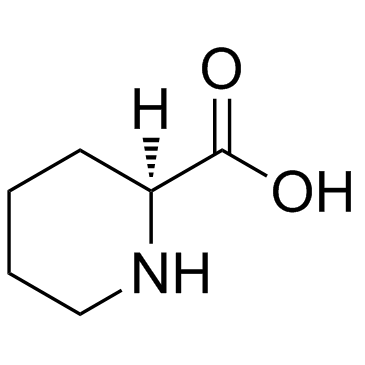 CAS#:3105-95-1
CAS#:3105-95-1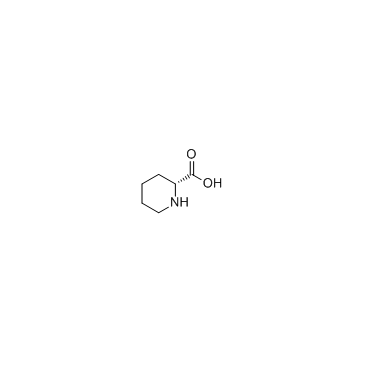 CAS#:1723-00-8
CAS#:1723-00-8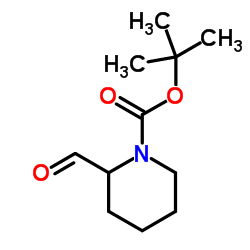 CAS#:157634-02-1
CAS#:157634-02-1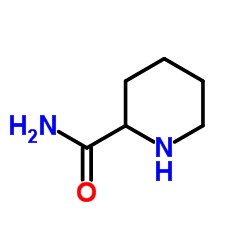 CAS#:19889-77-1
CAS#:19889-77-1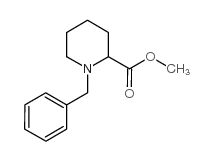 CAS#:124619-69-8
CAS#:124619-69-8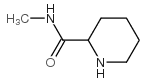 CAS#:53941-92-7
CAS#:53941-92-7
