L(-)-Pipecolinic acid
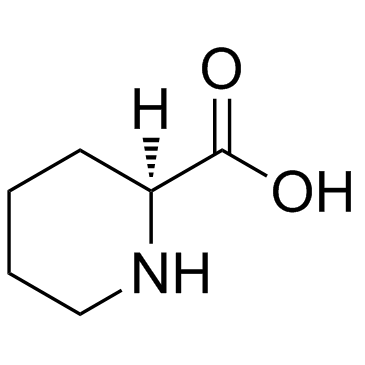
L(-)-Pipecolinic acid structure
|
Common Name | L(-)-Pipecolinic acid | ||
|---|---|---|---|---|
| CAS Number | 3105-95-1 | Molecular Weight | 129.157 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 265.8±33.0 °C at 760 mmHg | |
| Molecular Formula | C6H11NO2 | Melting Point | 272ºC | |
| MSDS | Chinese USA | Flash Point | 114.5±25.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of L(-)-Pipecolinic acidH-HoPro-OH is a breakdown product of lysine, accumulates in body fluids of infants with generalized genetic peroxisomal disorders, such as Zellweger syndrome, neonatal adrenoleukodystrophy. |
| Name | L-pipecolate |
|---|---|
| Synonym | More Synonyms |
| Description | H-HoPro-OH is a breakdown product of lysine, accumulates in body fluids of infants with generalized genetic peroxisomal disorders, such as Zellweger syndrome, neonatal adrenoleukodystrophy. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 265.8±33.0 °C at 760 mmHg |
| Melting Point | 272ºC |
| Molecular Formula | C6H11NO2 |
| Molecular Weight | 129.157 |
| Flash Point | 114.5±25.4 °C |
| Exact Mass | 129.078979 |
| PSA | 49.33000 |
| LogP | 0.00 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.479 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933399090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Three-dimensional quantitative structure-activity relationship analyses of substrates of the human proton-coupled amino acid transporter 1 (hPAT1).
Bioorg. Med. Chem. 19 , 6409-18, (2011) The proton-coupled amino acid transporter hPAT1 has recently gained much interest due to its ability to transport small drugs thereby allowing their oral administration. A three-dimensional quantitati... |
|
|
Lysine catabolism in Haemonchus contortus and Teladorsagia circumcincta.
Exp. Parasitol. 131(1) , 101-6, (2012) Catabolism of lysine through the pipecolate, saccharopine and cadaverine pathways has been investigated in L3 and adult Haemonchus contortus and Teladorsagia circumcincta. Both enzymes of the saccharo... |
|
|
Characterization of the nocardiopsin biosynthetic gene cluster reveals similarities to and differences from the rapamycin and FK-506 pathways.
ChemBioChem. 16(6) , 990-7, (2015) Macrolide-pipecolate natural products, such as rapamycin (1) and FK-506 (2), are renowned modulators of FK506-binding proteins (FKBPs). The nocardiopsins, from Nocardiopsis sp. CMB-M0232, are the newe... |
| (S)-piperidine-2-carboxylic acid |
| PIP-OH |
| L-PIPECOLATE |
| H-HOMOPRO-OH |
| (2S)-piperidine-2-carboxylic acid |
| (S)-2-Piperidinecarboxylic acid |
| Pipecolinic acid |
| 6-CARBOXYPIPERIDINE |
| DL-2-Piperidinecarboxylic acid |
| Pipecolic acid |
| α-Pipecolinic acid |
| a-Pipecolinic acid |
| HOMOPROLINE |
| L-HOMOPRO |
| L-Homoproline |
| (2S)-2-Piperidinecarboxylic acid |
| h-pip-oh |
| 2-Piperidinecarboxylic acid, (2S)- |
| H-L-PIP-OH |
| (S)-Pipecolinic acid |
| (±)-2-Piperidinecarboxylic acid |
| 2-Piperidinecarboxylic acid |
| (S)-(−)-2-Piperidinecarboxylic acid L-Homoproline |
| (L)-Pipecolic acid |
| (S)-(-)-2-PIPERIDINECARBOXYLIC ACID |
| L-Pipecolinic acid |
| EINECS 221-462-1 |
| L-Pipecolic Acid |
| Pipecolic acid, (S)-(-)- |
| Dihydrobaikiaine |
| UNII:H254GW7PVV |
| (S)-pipecolic acid |
| L(-)-Pipecolinic acid |
| DL-Pipecolinicacid |
| (S)-(−)-2-Piperidinecarboxylic acid |
| (2S)-piperidinium-2-carboxylate |
| (±)-Pipecolinic acid |
| MFCD00005981 |
| 2-Piperidinecarboxylic acid, (S)- |
| (±)-Pipecolic acid |
| 2-Carboxypiperidine |
| L-pipecolic acid zwitterion |
| piperidine-2-carboxylic acid |
| H-HOPRO-OH |
| L(-)-Pipecolinic acid |
 CAS#:56-87-1
CAS#:56-87-1 CAS#:28697-11-2
CAS#:28697-11-2 CAS#:923-27-3
CAS#:923-27-3 CAS#:4043-87-2
CAS#:4043-87-2 CAS#:500131-75-9
CAS#:500131-75-9![(4S,9aS)-4-phenylhexahydropyrido[2,1-c][1,4]oxazin-1-one Structure](https://image.chemsrc.com/caspic/495/290333-69-6.png) CAS#:290333-69-6
CAS#:290333-69-6 CAS#:625824-32-0
CAS#:625824-32-0![(4R,9aS)-4-Phenylhexahydropyrido[2,1-c][1,4]oxazin-1-one Structure](https://image.chemsrc.com/caspic/326/158221-57-9.png) CAS#:158221-57-9
CAS#:158221-57-9 CAS#:56099-73-1
CAS#:56099-73-1 CAS#:657-27-2
CAS#:657-27-2 CAS#:210533-45-2
CAS#:210533-45-2 CAS#:212557-00-1
CAS#:212557-00-1 CAS#:27262-40-4
CAS#:27262-40-4 CAS#:130606-00-7
CAS#:130606-00-7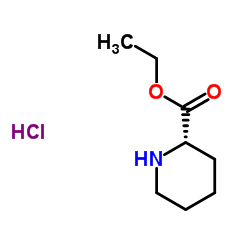 CAS#:123495-48-7
CAS#:123495-48-7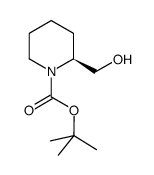 CAS#:134441-93-3
CAS#:134441-93-3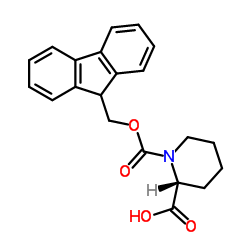 CAS#:101555-63-9
CAS#:101555-63-9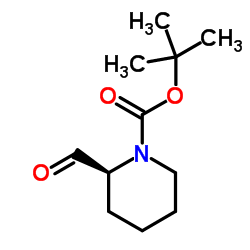 CAS#:150521-32-7
CAS#:150521-32-7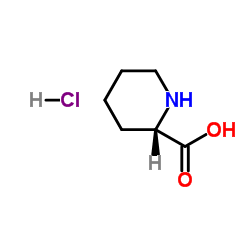 CAS#:2133-33-7
CAS#:2133-33-7
