6-tert-Butyl-2,3-naphthalenedicarbonitrile
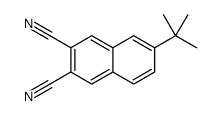
6-tert-Butyl-2,3-naphthalenedicarbonitrile structure
|
Common Name | 6-tert-Butyl-2,3-naphthalenedicarbonitrile | ||
|---|---|---|---|---|
| CAS Number | 32703-82-5 | Molecular Weight | 234.29600 | |
| Density | 1.11g/cm3 | Boiling Point | 429.7ºC at 760 mmHg | |
| Molecular Formula | C16H14N2 | Melting Point | 185-189ºC(lit.) | |
| MSDS | USA | Flash Point | 206.6ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 6-tert-Butyl-2,3-naphthalenedicarbonitrileBRD9876 is the “rigor” inhibitor that locks kinesin-5 (Eg5) in a state with enhanced microtubules (MTs) binding, leading to bundling and stabilization of MTs. BRD9876 interacts with the tyrosine 104 residue that is part of the α4-α6 allosteric binding pocket. BRD9876 specifically targets microtubule-bound Eg5 and selectively inhibits myeloma over CD34 cells. BRD9876 has the potential for multiple myeloma (MM) research[1][2][3][4]. |
| Name | 6-tert-Butyl-2,3-naphthalenedicarbonitrile |
|---|---|
| Synonym | More Synonyms |
| Description | BRD9876 is the “rigor” inhibitor that locks kinesin-5 (Eg5) in a state with enhanced microtubules (MTs) binding, leading to bundling and stabilization of MTs. BRD9876 interacts with the tyrosine 104 residue that is part of the α4-α6 allosteric binding pocket. BRD9876 specifically targets microtubule-bound Eg5 and selectively inhibits myeloma over CD34 cells. BRD9876 has the potential for multiple myeloma (MM) research[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | BRD9876 (10 μM; 24 hours) reveales rapid arrest of cells at the G2/M phase starting as early as 2h of treatment in MM1S cells[1]. BRD9876 exhibits approximately 3-fold selectivity for MM1S myeloma cells (IC50=3.1 μM) over CD34+ derived hematopoietic cells (IC50=9.1 μM)[1]. BRD9876 (0.1, 1, 10, 100 uM) is able to overcome, in MM1S cells, stromal resistance of bone marrow stromal cells (BMSCs) from MM bone marrow aspirates but only minimal effects are observed with BRD9876 against primary MM cells[1]. BRD9876 is completely ineffective at inhibiting the basal ATPase activity of Eg5, in contrast to loop L5-binding monastrol or α4/α6-binding BI8 which shows greater activity against basal Eg5 ATPase activity[1]. Cell Cycle Analysis[1] Cell Line: MM1S cells and CD34 hematopoietic cells Concentration: 10 μM Incubation Time: 24 hours Result: Revealed rapid arrest of cells at the G2/M phase starting as early as 2h of treatment in MM1S cells. Showed markedly less G2/M arrest in CD34 hematopoietic cells. |
| References |
| Density | 1.11g/cm3 |
|---|---|
| Boiling Point | 429.7ºC at 760 mmHg |
| Melting Point | 185-189ºC(lit.) |
| Molecular Formula | C16H14N2 |
| Molecular Weight | 234.29600 |
| Flash Point | 206.6ºC |
| Exact Mass | 234.11600 |
| PSA | 47.58000 |
| LogP | 3.88066 |
| Index of Refraction | 1.603 |
| InChIKey | MKILROQBJOOZKC-UHFFFAOYSA-N |
| SMILES | CC(C)(C)c1ccc2cc(C#N)c(C#N)cc2c1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | 20/21/22 |
| Safety Phrases | 36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2926909090 |
|
~44% 
6-tert-Butyl-2,... CAS#:32703-82-5 |
| Literature: Plater; Jeremiah; Bourhill Journal of the Chemical Society. Perkin Transactions 1, 2002 , vol. 2, # 1 p. 91 - 96 |
|
~% 
6-tert-Butyl-2,... CAS#:32703-82-5 |
| Literature: Plater; Jeremiah; Bourhill Journal of the Chemical Society. Perkin Transactions 1, 2002 , vol. 2, # 1 p. 91 - 96 |
|
~% 
6-tert-Butyl-2,... CAS#:32703-82-5 |
| Literature: Kovshev,E.I. et al. Journal of Organic Chemistry USSR (English Translation), 1971 , vol. 7, p. 364 - 366 Zhurnal Organicheskoi Khimii, 1971 , vol. 7, # 2 p. 369 - 371 |
| HS Code | 2926909090 |
|---|---|
| Summary | HS:2926909090 other nitrile-function compounds VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
| 6-tert-butylnaphthalene-2,3-dicarbonitrile |
| MFCD00209558 |

 CAS#:105011-00-5
CAS#:105011-00-5![6-tert-butyl-3-iminobenzo[f]isoindol-1-amine structure](https://image.chemsrc.com/caspic/337/159454-81-6.png) CAS#:159454-81-6
CAS#:159454-81-6 CAS#:58687-99-3
CAS#:58687-99-3 CAS#:39049-43-9
CAS#:39049-43-9
