BAY 57-1293
Modify Date: 2025-08-23 20:33:18
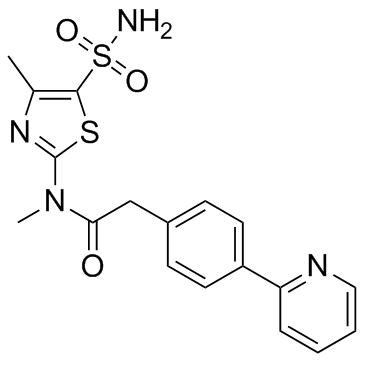
BAY 57-1293 structure
|
Common Name | BAY 57-1293 | ||
|---|---|---|---|---|
| CAS Number | 348086-71-5 | Molecular Weight | 402.491 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 639.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C18H18N4O3S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 340.5±34.3 °C | |
Use of BAY 57-1293BAY 57-1293 represents a new class of potent inhibitors of herpes simplex virus (HSV) that target the virus helicase primase complex.IC50 Value: 20 nM (HSV-1) [1]Target: HSVin vitro: BAY 57-1293 is nearly two orders of magnitude more potent than acyclovirin vitro and the superiority was even more prominent when the viral load was increased (BAY 57-1293 IC50 = 12 nM, 20 nM and 50 nM; acyclovir IC50 = 1uM, 3M and 10 50 uM at a multiplicity of infection (m.o.i.) of 0.0025, 0.02 and 0.2, respectively). A minor increase in IC50 values at higher viral loads was observed for all thiazolyl compounds listed in Table 1. BAY 57-1293 was also active against porcine (IC50 = 5 uM) and bovine (IC50 = 0.12 uM) herpes strains [1].in vivo: Delayed treatment with BAY 57-1293 (20 mg/kg 2× daily per os, treatment day 4-14) abrogates progression of disease symptoms (mean of 10 animals per group) of HSV-2 infected guinea pigs within 1 d of treatment and healing is observed subsequently, whereas a 7.5 fold higher dose of valacyclovir (150 mg/kg 2× daily) shows marginal therapeutic efficacy compared with placebo [1]. The compound given orally, or intraperitoneally once per day at a dose of 15 mg/kg for 4 successive days was equally effective or superior to a much higher dose of famciclovir (1mg/ml, i.e. approximately 140-200mg/kg/day) given in the drinking water for 7 consecutive days, which, in our hands, is the most effective method for administering famciclovir to mice [2].Toxicity: Exploratory toxicology and safety pharmacology studies did not reveal any safety relevant findings at 30, 100 and 300 mg/kg BAY 57-1293 (once daily per os) in a 4-week chronic toxicity study in dogs. However, administration of the same dose to rats for 4 weeks resulted in a dose-dependent transitional hyperplasia of the urinary bladder epithelium [1]. |
| Name | N-methyl-N-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-(4-pyridin-2-ylphenyl)acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | BAY 57-1293 represents a new class of potent inhibitors of herpes simplex virus (HSV) that target the virus helicase primase complex.IC50 Value: 20 nM (HSV-1) [1]Target: HSVin vitro: BAY 57-1293 is nearly two orders of magnitude more potent than acyclovirin vitro and the superiority was even more prominent when the viral load was increased (BAY 57-1293 IC50 = 12 nM, 20 nM and 50 nM; acyclovir IC50 = 1uM, 3M and 10 50 uM at a multiplicity of infection (m.o.i.) of 0.0025, 0.02 and 0.2, respectively). A minor increase in IC50 values at higher viral loads was observed for all thiazolyl compounds listed in Table 1. BAY 57-1293 was also active against porcine (IC50 = 5 uM) and bovine (IC50 = 0.12 uM) herpes strains [1].in vivo: Delayed treatment with BAY 57-1293 (20 mg/kg 2× daily per os, treatment day 4-14) abrogates progression of disease symptoms (mean of 10 animals per group) of HSV-2 infected guinea pigs within 1 d of treatment and healing is observed subsequently, whereas a 7.5 fold higher dose of valacyclovir (150 mg/kg 2× daily) shows marginal therapeutic efficacy compared with placebo [1]. The compound given orally, or intraperitoneally once per day at a dose of 15 mg/kg for 4 successive days was equally effective or superior to a much higher dose of famciclovir (1mg/ml, i.e. approximately 140-200mg/kg/day) given in the drinking water for 7 consecutive days, which, in our hands, is the most effective method for administering famciclovir to mice [2].Toxicity: Exploratory toxicology and safety pharmacology studies did not reveal any safety relevant findings at 30, 100 and 300 mg/kg BAY 57-1293 (once daily per os) in a 4-week chronic toxicity study in dogs. However, administration of the same dose to rats for 4 weeks resulted in a dose-dependent transitional hyperplasia of the urinary bladder epithelium [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 639.4±65.0 °C at 760 mmHg |
| Molecular Formula | C18H18N4O3S2 |
| Molecular Weight | 402.491 |
| Flash Point | 340.5±34.3 °C |
| Exact Mass | 402.082031 |
| PSA | 142.87000 |
| LogP | 0.91 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.648 |
| UNII-07HQ1TJ4JE |
| BAY 57-1293 |
| N-Methyl-N-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-[4-(2-pyridinyl)phenyl]acetamide |
| Benzeneacetamide, N-[5-(aminosulfonyl)-4-methyl-2-thiazolyl]-N-methyl-4-(2-pyridinyl)- |
| Pritelivir |
| Benzeneacetamide,N-(5-(aminosulfonyl)-4-methyl-2-thiazolyl)-N-methyl-4-(2-pyridinyl) |