Boc-Cyclolencine
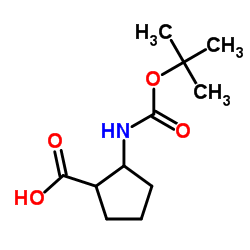
Boc-Cyclolencine structure
|
Common Name | Boc-Cyclolencine | ||
|---|---|---|---|---|
| CAS Number | 35264-09-6 | Molecular Weight | 229.27 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 382.5±31.0 °C at 760 mmHg | |
| Molecular Formula | C11H19NO4 | Melting Point | 130-135 °C | |
| MSDS | Chinese USA | Flash Point | 185.1±24.8 °C | |
Use of Boc-CyclolencineBoc-Cycloleucine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 1-[(2-methylpropan-2-yl)oxycarbonylamino]cyclopentane-1-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Boc-Cycloleucine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 382.5±31.0 °C at 760 mmHg |
| Melting Point | 130-135 °C |
| Molecular Formula | C11H19NO4 |
| Molecular Weight | 229.27 |
| Flash Point | 185.1±24.8 °C |
| Exact Mass | 229.131409 |
| PSA | 75.63000 |
| LogP | 1.47 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.495 |
| InChIKey | YBZCSKVLXBOFSL-UHFFFAOYSA-N |
| SMILES | CC(C)(C)OC(=O)NC1(C(=O)O)CCCC1 |
| Water Solubility | Soluble in hot water |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
New alpha-thiol dipeptide dual inhibitors of angiotensin-I converting enzyme and neutral endopeptidase EC 3.4.24.11.
J. Med. Chem. 38 , 5023, (1995) Dual inhibitors of the two zinc metallopeptidases, neutral endopeptidase (NEP, EC 3.4.24.11) and angiotensin-I converting enzyme, have been the focus of much clinical interest for the treatment of hyp... |
|
|
Conformational versatility of the N alpha-acylated tripeptide amide tail of oxytocin. Synthesis and crystallographic characterization of three C2 alpha-backbone modified, conformationally restricted analogues.
Int. J. Pept. Protein Res. 42 , 459, (1993) The synthesis, physical and analytical characterization, and crystal-state structural analysis by X-ray diffraction of three analogues of the N alpha-acylated tripeptide amide tail of oxytocin, each c... |
| Boc-1-aminocyclopentane-1-carboxylic acid |
| Boc-Cycloleucine |
| 1-(tert-Butoxycarbonylamino)cyclopentanecarboxylic Acid |
| Cyclopentanecarboxylic acid, 1-[[(1,1-dimethylethoxy)carbonyl]amino]- |
| 1-({[(2-Methyl-2-propanyl)oxy]carbonyl}amino)cyclopentanecarboxylic acid |
| Cyclopentanecarboxylic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]- |
| N-Boc-1-amino-1-cyclopentanecarboxylic acid |
| 2-tert-Butoxycarbonylamino-cyclopentanecarboxylic acid |
| MFCD01076126 |
| 1-N-Boc-Aminocyclopentanecarboxylic Acid |
| 1-N-Boc-Aminocyclopentanecarboxylicacid |
| Boc-Cyclolencine |
| N-Boc-cycloleucine |
| 1-[(tert-Butoxycarbonyl)amino]cyclopentanecarboxylic acid |
| 1-(Boc-amino)cyclopentanecarboxylic acid |
| 2-({[(2-Methyl-2-propanyl)oxy]carbonyl}amino)cyclopentanecarboxylic acid |
| 1-((tert-Butoxycarbonyl)amino)cyclopentanecarboxylic acid |
 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:52-52-8
CAS#:52-52-8 CAS#:58632-95-4
CAS#:58632-95-4 CAS#:123-91-1
CAS#:123-91-1 CAS#:74651-77-7
CAS#:74651-77-7![1,3-Diazaspiro[4.4]nonane-2,4-dione Structure](https://image.chemsrc.com/caspic/354/699-51-4.png) CAS#:699-51-4
CAS#:699-51-4 CAS#:120-92-3
CAS#:120-92-3 CAS#:1070-19-5
CAS#:1070-19-5 CAS#:5471-59-0
CAS#:5471-59-0
