MST312
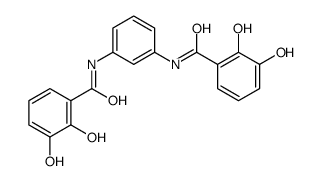
MST312 structure
|
Common Name | MST312 | ||
|---|---|---|---|---|
| CAS Number | 368449-04-1 | Molecular Weight | 380.35100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C20H16N2O6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS09 |
Signal Word | Warning | |
Use of MST312MST-312 is a telomerase inhibitor. MST-312 is a chemically modified derivative of green tea epigallocatechin gallate (EGCG). MST-312 can be used for the research of cancer, such as multiple myeloma (MM)[1]. |
| Name | N-[3-[(2,3-dihydroxybenzoyl)amino]phenyl]-2,3-dihydroxybenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | MST-312 is a telomerase inhibitor. MST-312 is a chemically modified derivative of green tea epigallocatechin gallate (EGCG). MST-312 can be used for the research of cancer, such as multiple myeloma (MM)[1]. |
|---|---|
| Related Catalog | |
| Target |
telomeras[1] |
| In Vitro | MST-312 (2~8 μM; 0~72 hours; U-266 cells) reduces cellular viability in a dose dependent and time-dependent manner[1]. MST-312 (2~8 μM; 48 hours; U-266 cells) induces cell apoptosis in a dose-dependent manner[1]. MST-312 (2 μM; 48 hours; U-266 cells) up-regulates the pro-apoptotic gene Bax and down-regulates the anti-apoptotic gene Bcl-2 and suppresses the expression of c-Myc and hTERT genes[1]. Cell Viability Assay[1] Cell Line: U-266 cells Concentration: 2~8 μM Incubation Time: 0~72 hours Result: The viability of U-266 cells was substantially decreased in a dose dependent and time-dependent manner, in response to exposure to MST-312. Apoptosis Analysis[1] Cell Line: U-266 cells Concentration: 2~8 μM Incubation Time: 48 hours Result: Induced cell apoptosis in a dose-dependent manner. RT-PCR[1] Cell Line: U-266 cells Concentration: 2 μM Incubation Time: 48 hours Result: Up-regulated the pro-apoptotic gene Bax and down-regulated the anti-apoptotic gene Bcl-2 and suppressed the expression of c-Myc and hTERT genes. |
| References |
| Molecular Formula | C20H16N2O6 |
|---|---|
| Molecular Weight | 380.35100 |
| Exact Mass | 380.10100 |
| PSA | 146.10000 |
| LogP | 3.78160 |
| InChIKey | MIQUEZGHEJGPJB-UHFFFAOYSA-N |
| SMILES | O=C(Nc1cccc(NC(=O)c2cccc(O)c2O)c1)c1cccc(O)c1O |
| Storage condition | 2-8°C |
|
~% 
MST312 CAS#:368449-04-1 |
| Literature: Caulder; Brueckner; Powers; Koenig; Parac; Leary; Raymond Journal of the American Chemical Society, 2001 , vol. 123, # 37 p. 8923 - 8938 |
|
~% 
MST312 CAS#:368449-04-1 |
| Literature: Caulder; Brueckner; Powers; Koenig; Parac; Leary; Raymond Journal of the American Chemical Society, 2001 , vol. 123, # 37 p. 8923 - 8938 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
MST-312 Alters Telomere Dynamics, Gene Expression Profiles and Growth in Human Breast Cancer Cells.
J. Nutrigenet. Nutrigenomics 7 , 283-98, (2015) Targeting telomerase is a potential cancer management strategy given that it allows unlimited cellular replication in the majority of cancers. Dysfunctional telomeres are recognized as double-strand b... |
|
|
Fluorescence-based duplex-quadruplex competition test to screen for telomerase RNA quadruplex ligands.
Nucleic Acids Res. 39 , e21, (2011) RNA and DNA guanine-rich sequences can adopt unusual structures called Guanine quadruplexes (G4). A quadruplex-prone RNA sequence is present at the 5'-end of the 451-nt-long RNA component of telomeras... |
| telomerase inhibitor IX |
| IN1063 |
| N,N'-1,3-Phenylenebis-[2,3-dihydroxy-benzamide] |
| N,N'-bis(2,3-dihydroxybenzoyl)-1,3-phenylenediamine |
| N,N-1,3-phenylenebis-(2,3-dihydroxybenzamide) |
| MST-312 |
| 1,3-bis(2,3-dihydroxybenzamido)benzene |
| Benzamide,N,N'-1,3-phenylenebis[2,3-dihydroxy |
| MST312 |