Maraviroc
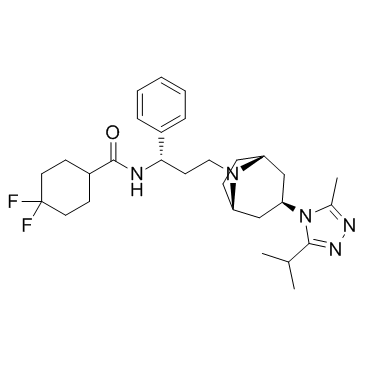
Maraviroc structure
|
Common Name | Maraviroc | ||
|---|---|---|---|---|
| CAS Number | 376348-65-1 | Molecular Weight | 513.666 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C29H41F2N5O | Melting Point | 79-81ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of MaravirocMaraviroc is a selective CCR5 antagonist with activity against human HIV. |
| Name | maraviroc |
|---|---|
| Synonym | More Synonyms |
| Description | Maraviroc is a selective CCR5 antagonist with activity against human HIV. |
|---|---|
| Related Catalog | |
| Target |
MIP-1α-CCR5:3.3 nM (IC50, in HEK-293 cell membrane) RANTES-CCR5:5.2 nM (IC50, in HEK-293 cell membrane) MIP-1β-CCR5:7.2 nM (IC50, in HEK-293 cell membrane) HIV-1 (Ba-L):1.1 nM (IC50, in PM-1 cells) |
| In Vitro | Maraviroc is a selective CCR5 antagonist with potent anti-human immunodeficiency virus type 1 (HIV-1) activity. Maraviroc inhibits the downstream event of chemokine-induced intracellular calcium redistribution, with IC50s ranging from 7 to 30 nM obtained against MIP-1β, MIP-1α and RANTES. Maraviroc is active (IC90) at low nanomolar concentrations against HIV-1 Ba-L (a lab-adapted R5 strain) when measured in a 5-day antiviral assay using either isolated multiple (pooled) donor PBMC (IC90, 3.1 nM), single-donor PBMC (IC90, 1.8 nM) or PM-1 cells (IC90, 1.1 nM)[1]. |
| In Vivo | Clearance values are moderate to high in both rat and dog species following i.v. administration (74 and 21 mL/min/kg, respectively). Maraviroc also has a moderate volume of distribution in both species (4.3 to 6.5 liters/kg). The half-life values of maraviroc are 0.9 h in the rat and 2.3 h in the dog. Following oral administration (2 mg/kg) to the dog, the Cmax(256 ng/mL) occurs 1.5 h. post-dose, and the bioavailability is 40%. For the rat, investigation of the concentrations obtain in the portal vein following oral administration indicated that approximately 30% of the administered dose is absorbed from the intestinal tract[1]. In the DSS/TNBS colitis and in the transfer model, Maraviroc attenuates development of intestinal inflammation by selectively reducing the recruitment of CCR5 bearing leukocytes[2] |
| Kinase Assay | Binding of 125I-labeled MIP-1α, MIP-1β, and RANTES to CCR5 is measured essentially using intact HEK-293 cells stably expressing the receptor or membrane preparations thereof. Briefly, cells are resuspended in binding buffer (50 mM HEPES containing 1 mM CaCl2, 5 mM MgCl2, and 0.5% bovine serum albumin [BSA] and adjusted to pH 7.4) to a density of 2×106 cells/mL. For membrane preparations, phosphate-buffered saline (PBS)-washed cells are resuspended in lysis buffer (20 mM HEPES, 1 mM CaCl2, 1 tablet COMPLETE per 50 mL, pH 7.4; Boehringer) prior to homogenization in a Polytron hand-held homogenizer, ultracentrifugation (40,000× g for 30 min), and resuspension in binding buffer to a protein concentration of 0.25 mg/mL (12.5 μg of membrane protein is used in each well of a 96-well plate). 125I-radiolabeled MIP-1α, MIP-1β, and RANTES are prepared and diluted in binding buffer to a final concentration of 400 pM in the assay. Appropriate maraviroc dilutions are added to each well to a final volume of 100 μL, the assay plates incubated for 1 h, and the contents filtered through preblocked and washed Unifilter plates which are counted following overnight drying[1]. |
| Cell Assay | HEK-293 cell aliquots (100 μL at 1×106 cells/mL) are plated into poly-D-lysine-coated plates and incubated at 37°C overnight. A 1:1 mix of soluble recombinant human CD4 (sCD4) (diluted to 4.5 nM in culture medium) and HIV-1 gp120 is incubated at room temperature for 15 min prior to its addition to PBS-washed cells in the presence of dilutions of maraviroc to enable IC50 determination. The assay plates are incubated at 37°C for 1 h and washed. Eu3+-labeled anti-gp120 antibody (1/500 dilution in assay buffer) is added to each well (50 μL) and incubated for 1 h. The plate is washed three times with wash buffer prior to the addition of enhancement solution (200 μL/well) and measurement of Eu3+ fluorescence (Victor2multilabel counter; “Europium” protocol). Nonspecific binding is taken as the fluorescence measured for gp120 incubated with cells in the absence of preincubation with sCD4[1]. |
| Animal Admin | Rats and Dogs[1] Preclinical pharmacokinetic studies are carried out with maraviroc following a single intravenous and oral administration to both male Sprague-Dawley rats (1 mg/kg of body weight given intravenously [i.v.] and 10 mg/kg given orally [p.o.]; n=2) and male beagle dogs (0.5 mg/kg i.v. and 2 mg/kg p.o; n=4). Plasma samples are taken for up to 24 h postdose, and the concentrations of unchanged maraviroc are determined using a specific high-performance liquid chromatography-tandem mass spectrum assay. Mice[2] Splenocytes are collected from 6-10 week old CCR5-/- mice or wild-type controlmice (n=8 per group) and naive CD4+ CD45RBhigh T-cells are isolated by cell sorting. A total of 3×105 CD45RBhigh cells are then injected intravenously into Rag1-/- mice that are subsequently weighed and assessed for fecal score every 20 days to evaluate IBD development. To investigate whether Maraviroc rescues from intestinal inflammation induced by transfer colitis, Rag1-/- mice are injected with CD4+ CD45RB-/- T-cells and 34 days later randomized into either a control group (no further treatment, n=6) or treatment with Maraviroc, 50 mg/kg/d Maraviroc per os (n=4) for 3 weeks, 5 d/week. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 79-81ºC |
| Molecular Formula | C29H41F2N5O |
| Molecular Weight | 513.666 |
| Exact Mass | 513.327942 |
| PSA | 63.05000 |
| LogP | 3.60 |
| Index of Refraction | 1.628 |
| Storage condition | Store at +4°C |
| Precursor 10 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Serum amyloid A chemoattracts immature dendritic cells and indirectly provokes monocyte chemotaxis by induction of cooperating CC and CXC chemokines.
Eur. J. Immunol. 45(1) , 101-12, (2015) Serum amyloid A (SAA) is an acute phase protein that is upregulated in inflammatory diseases and chemoattracts monocytes, lymphocytes, and granulocytes via its G protein-coupled receptor formyl peptid... |
|
|
Procoagulant and proinflammatory effects of red blood cells on lipopolysaccharide-stimulated monocytes.
J. Thromb. Haemost. 13 , 1676-82, (2015) We aimed to evaluate the mechanisms underlying the effects of red blood cells (RBCs) on the reactivity of monocytes to lipopolysaccharide (LPS) stimulation.Measurements of tissue factor (TF) antigen a... |
|
|
Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection.
Antimicrob. Agents Chemother. 59(1) , 397-406, (2014) GSK1265744 is a new HIV integrase strand transfer inhibitor (INSTI) engineered to deliver efficient antiviral activity with a once-daily, low-milligram dose that does not require a pharmacokinetic boo... |
| 4,4-Difluoro-N-{(1S)-3-[(1R,5S)-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl}cyclohexanecarboxamide |
| Maraviroc |
| 4,4-difluoro-N-[(1S)-3-{(1R,5S)-3-[3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]oct-8-yl}-1-phenylpropyl]cyclohexanecarboxamide |
| exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide |
| maroviroc |
| Celsentri |
| Cyclohexanecarboxamide, 4,4-difluoro-N-[(1S)-3-[(3-exo)-3-[3-methyl-5-(1-methylethyl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl]- |
| Cyclohexanecarboxamide, 4,4-difluoro-N-((1S)-3-((3-exo)-3-(3-methyl-5-(1-methylethyl)-4H-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)oct-8-yl)-1-phenylpropyl)- |
| Cyclohexanecarboxamide, 4,4-difluoro-N-[(1S)-3-[(1R,5S)-3-[3-methyl-5-(1-methylethyl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl]- |
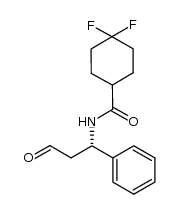 CAS#:376348-78-6
CAS#:376348-78-6![3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane 4-methylbenzenesulfonate Structure](https://image.chemsrc.com/caspic/483/423165-08-6.png) CAS#:423165-08-6
CAS#:423165-08-6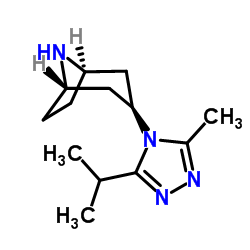 CAS#:423165-07-5
CAS#:423165-07-5![(1S)-3-[3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-exo-8-azabicyclo[3.2.1]oct-8-yl]-1-phenyl-1-propanamine Structure](https://image.chemsrc.com/caspic/298/376348-71-9.png) CAS#:376348-71-9
CAS#:376348-71-9 CAS#:376348-75-3
CAS#:376348-75-3 CAS#:122665-97-8
CAS#:122665-97-8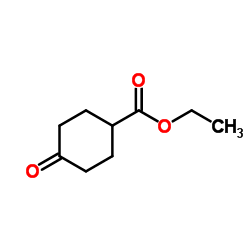 CAS#:17159-79-4
CAS#:17159-79-4 CAS#:178312-47-5
CAS#:178312-47-5![((S)-3-[3-(3-ISOPROPYL-5-METHYL-[1,2,4]TRIAZOL-4-YL)-8-AZA-BICYCLO[3.2.1]OCT-8-YL]-1-PHENYL-PROPYL)-CARBAMIC ACID TERT-BUTYL ESTER Structure](https://image.chemsrc.com/caspic/336/376348-70-8.png) CAS#:376348-70-8
CAS#:376348-70-8 CAS#:37088-66-7
CAS#:37088-66-7