7α-Hydroxy-4-cholesten-3-one
Modify Date: 2024-01-02 18:24:58
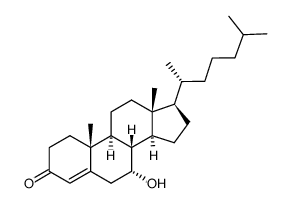
7α-Hydroxy-4-cholesten-3-one structure
|
Common Name | 7α-Hydroxy-4-cholesten-3-one | ||
|---|---|---|---|---|
| CAS Number | 3862-25-7 | Molecular Weight | 400.63700 | |
| Density | 1.03g/cm3 | Boiling Point | 516.7ºC at 760 mmHg | |
| Molecular Formula | C27H44O2 | Melting Point | 182-184ºC | |
| MSDS | N/A | Flash Point | 218.5ºC | |
Use of 7α-Hydroxy-4-cholesten-3-one7α-Hydroxy-4-cholesten-3-one is an intermediate in synthesis of bile acids from cholesterol. 7α-Hydroxy-4-cholesten-3-one is a pregnane X receptor (PXR) agonist. 7α-Hydroxy-cholest-4-en-3-one is a biomarker for bile acid loss, irritable bowel syndrome, and other diseases associated with defective bile acid biosynthesis. 7α-Hydroxy-cholest-4-en-3-one is the physiological substrate for CYP8B1[1][2]. |
| Name | 7α-hydroxycholest-4-en-3-one |
|---|---|
| Synonym | More Synonyms |
| Description | 7α-Hydroxy-4-cholesten-3-one is an intermediate in synthesis of bile acids from cholesterol. 7α-Hydroxy-4-cholesten-3-one is a pregnane X receptor (PXR) agonist. 7α-Hydroxy-cholest-4-en-3-one is a biomarker for bile acid loss, irritable bowel syndrome, and other diseases associated with defective bile acid biosynthesis. 7α-Hydroxy-cholest-4-en-3-one is the physiological substrate for CYP8B1[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Endogenous Metabolite[1] Pregnane X receptor (PXR)[1] |
| In Vitro | 7α-Hydroxy-4-cholesten-3-one is found relatively upstream in the biosynthetic pathway to bile acids (e.g. chenodeoxycholic acid). The first step is the incorporation of the 7α-hydroxy group onto cholesterol by cytochrome P450 7A1, and the second step is the oxidation and isomerization of the 3-hydroxy group and the Δ5,6-double bond by 3β-hydroxy steroid dehydrogenase to yield 7α-Hydroxy-4-cholesten-3-one. The deletion of the gene that expresses P450 27A1, which is found downstream in the bile acid pathway, results in the accumulation of the precursor, 7α-Hydroxy-4-cholesten-3-one[1]. |
| In Vivo | 7α-Hydroxy-4-cholesten-3-one strongly relates to the hepatic enzymatic activity of CYP7A1 at steady-state conditions as well as during the rapid diurnal changes that occur in the rat. That serum 7α-Hydroxy-4-cholesten-3-one has a pronounced diurnal rhythm[2]. |
| References |
| Density | 1.03g/cm3 |
|---|---|
| Boiling Point | 516.7ºC at 760 mmHg |
| Melting Point | 182-184ºC |
| Molecular Formula | C27H44O2 |
| Molecular Weight | 400.63700 |
| Flash Point | 218.5ºC |
| Exact Mass | 400.33400 |
| PSA | 37.30000 |
| LogP | 6.56770 |
| Index of Refraction | 1.53 |
| Storage condition | -20°C |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| 7alpha-Hydroxy-4-cholesten-3-one |
| Cholest-4-en-7alpha-ol-3-one |
| (7R,8S,9S,10R,13R,14S,17R)-7-hydroxy-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one |
| 7-Hydroxycholest-4-en-3-one |
| 7|A-Hydroxy-4-cholesten-3-one |
| 7alpha-Hydroxycholest-4-en-3-one |
| 7alpha-hydroxycholest-4-en-3-one |
| 7a-hydroxy-cholestene-3-one |
 CAS#:59783-78-7
CAS#:59783-78-7 CAS#:65851-87-8
CAS#:65851-87-8 CAS#:566-26-7
CAS#:566-26-7 CAS#:474-25-9
CAS#:474-25-9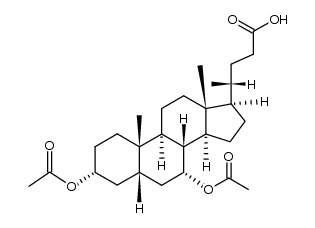 CAS#:33628-52-3
CAS#:33628-52-3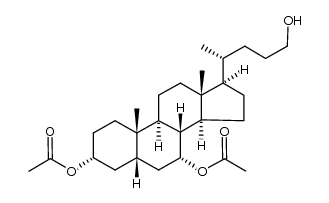 CAS#:107088-76-6
CAS#:107088-76-6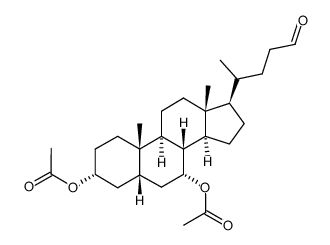 CAS#:81277-81-8
CAS#:81277-81-8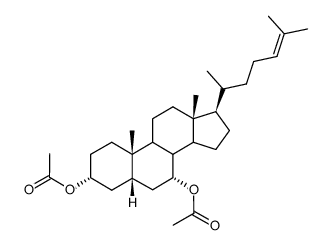 CAS#:66778-96-9
CAS#:66778-96-9 CAS#:120019-48-9
CAS#:120019-48-9