Bis(2,2,2-trichloroethyl)Azodicarboxylate
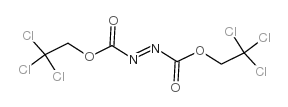
Bis(2,2,2-trichloroethyl)Azodicarboxylate structure
|
Common Name | Bis(2,2,2-trichloroethyl)Azodicarboxylate | ||
|---|---|---|---|---|
| CAS Number | 38857-88-4 | Molecular Weight | 380.82500 | |
| Density | 1.78g/cm3 | Boiling Point | 380.7ºC at 760 mmHg | |
| Molecular Formula | C6H4Cl6N2O4 | Melting Point | 109-111ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 184.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | Bis(2,2,2-trichloroethyl) Azodicarboxylate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.78g/cm3 |
|---|---|
| Boiling Point | 380.7ºC at 760 mmHg |
| Melting Point | 109-111ºC(lit.) |
| Molecular Formula | C6H4Cl6N2O4 |
| Molecular Weight | 380.82500 |
| Flash Point | 184.1ºC |
| Exact Mass | 377.83000 |
| PSA | 77.32000 |
| LogP | 4.45220 |
| Index of Refraction | 1.566 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2927000090 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2927000090 |
|---|---|
| Summary | 2927000090 other diazo-, azo- or azoxy-compounds。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Catalytic and enantioselective aza-ene and hetero-Diels-Alder reactions of alkenes and dienes with azodicarboxylates.
Org. Biomol. Chem. 3(12) , 2344-9, (2005) Lewis acids such as Cu(OTf)(2), Zn(OTf)(2), Yb(OTf)(3) and Nd(OTf)(3) catalyze the aza-ene reaction of alkenes with azodicarboxylates, giving the allylic amination adducts. The use of bis(2,2,2-trichl... |
|
|
Stereoselective formation of carbon-carbon bonds via SN2-displacement: synthesis of substituted cycloalkyl[b]indoles.
J. Org. Chem. 70(21) , 8385-94, (2005) A general asymmetric synthesis of substituted cycloalkyl[b]indoles has been accomplished. The key features of this approach are (1) the utilization of a Japp-Klingemann condensation/Fischer cyclizatio... |
|
|
A convenient synthesis of N-acetyllactosamine derivatives from lactal.
Carbohydr. Res. 247 , 159-64, (1993) In a thermal inverse-type hetero-Diels-Alder reaction of O-silyl-protected lactal 1 and bis(2,2,2-trichloroethyl) azodicarboxylate (2), the dihydrooxadiazine derivative 3 was obtained in a very high y... |
| 2,2,2-trichloroethyl N-(2,2,2-trichloroethoxycarbonylimino)carbamate |
| MFCD00010607 |
| Azodicarboxylic Acid Bis(2,2,2-trichloroethyl) Ester |
 CAS#:38858-02-5
CAS#:38858-02-5![2,4-dimethyl-(1rN,2tH,4tH,5cN,6cH,8cH)-3,7-dioxa-9,10-diaza-tetracyclo[3.3.2.02,4.06,8]decane-9,10-dicarboxylic acid bis-(2,2,2-trichloro-ethyl) ester structure](https://image.chemsrc.com/caspic/076/69496-46-4.png) CAS#:69496-46-4
CAS#:69496-46-4