Cyclopropylboronic acid

Cyclopropylboronic acid structure
|
Common Name | Cyclopropylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 411235-57-9 | Molecular Weight | 85.90 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 205.1±23.0 °C at 760 mmHg | |
| Molecular Formula | C3H7BO2 | Melting Point | 90-95 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 77.9±22.6 °C | |
| Symbol |



GHS05, GHS07, GHS08 |
Signal Word | Danger | |
Use of Cyclopropylboronic acidCyclopropaneboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Cyclopropylboronic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Cyclopropaneboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 205.1±23.0 °C at 760 mmHg |
| Melting Point | 90-95 °C(lit.) |
| Molecular Formula | C3H7BO2 |
| Molecular Weight | 85.90 |
| Flash Point | 77.9±22.6 °C |
| Exact Mass | 86.053909 |
| PSA | 40.46000 |
| LogP | 0.20 |
| Vapour Pressure | 0.1±0.9 mmHg at 25°C |
| Index of Refraction | 1.443 |
| InChIKey | WLVKDFJTYKELLQ-UHFFFAOYSA-N |
| SMILES | OB(O)C1CC1 |
| Storage condition | Store below -20 |
| Symbol |



GHS05, GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318-H360FD |
| Precautionary Statements | P201-P280-P305 + P351 + P338-P308 + P313 |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2942000000 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
|
Palladium-catalyzed decarboxylative coupling of isatoic anhydrides with arylboronic acids.
Org. Lett. 13 , 6114-6117, (2011) The decarboxylative coupling of isatoic anhydrides with arylboronic acids was realized for the first time in the presence of Pd(2)(dba)(3) and DPEphos, achieving aryl o-aminobenzoates with yields rang... |
|
|
Palladium-catalyzed alkylation of sp2 and sp3 C-H bonds with methylboroxine and alkylboronic acids: two distinct C-H activation pathways.
J. Am. Chem. Soc. 128 , 12634, (2006) Palladium-catalyzed alkylations of sp2 and sp3 C-H bonds with either methylboroxine or alkylboronic acids were developed. Ag2O or AgCO3 is used as a crucial oxidant and promoter for the transmetalatio... |
|
|
Copper-mediated N-cyclopropylation of azoles, amides, and sulfonamides by cyclopropylboronic acid.
J. Org. Chem. 73 , 6441, (2008) Reaction of azoles, amides, and sulfonamides in dichloroethane with readily available cyclopropylboronic acid in the presence of copper acetate and sodium carbonate afforded the N-cyclopropyl derivati... |
| MFCD04038750 |
| acide cyclopropylboronique |
| Cyclopropyl Boronic Acid |
| Boronic acid, B-cyclopropyl- |
| Cyclopropylboronic acid |
| Cyclopropaneboronic acid |
| cyclopropyl boronic |
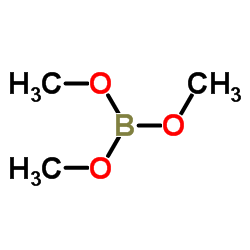

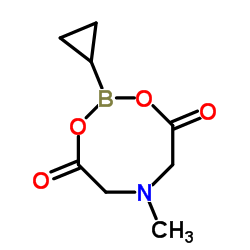


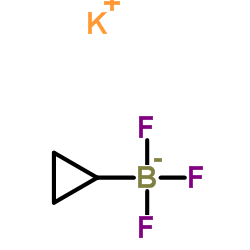
 CAS#:1034467-80-5
CAS#:1034467-80-5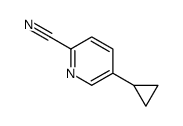 CAS#:188918-74-3
CAS#:188918-74-3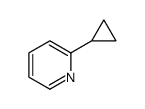 CAS#:20797-87-9
CAS#:20797-87-9 CAS#:366-18-7
CAS#:366-18-7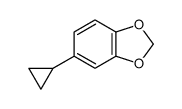 CAS#:29574-42-3
CAS#:29574-42-3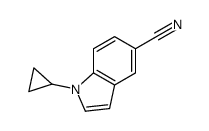 CAS#:256936-19-3
CAS#:256936-19-3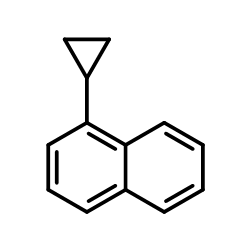 CAS#:25033-19-6
CAS#:25033-19-6 CAS#:30170-62-8
CAS#:30170-62-8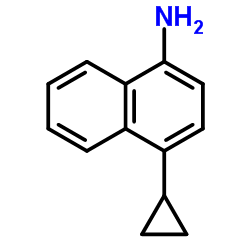 CAS#:878671-94-4
CAS#:878671-94-4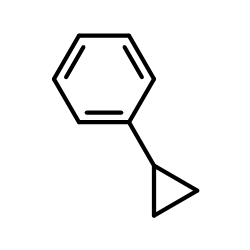 CAS#:873-49-4
CAS#:873-49-4
