CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
VS2851000
-
CHEMICAL NAME :
-
9,10-Secocholesta-5,7,10(19)-triene-1,3-diol, (1-alpha,3-beta,5Z,7E)-
-
CAS REGISTRY NUMBER :
-
41294-56-8
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
19
-
MOLECULAR FORMULA :
-
C27-H44-O2
-
MOLECULAR WEIGHT :
-
400.71
-
WISWESSER LINE NOTATION :
-
L56 FYTJ A1 BY1&3Y1&1 FU2U- AL6YYTJ BU1 CQ EQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
340 ug/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Olfaction) - effect, not otherwise specified Endocrine - other changes Skin and Appendages - hair
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 8,560,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
40 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PIXXD2 PCT (Patent Cooperation Treaty) International Application. (U.S. Patent and Trademark Office, Foreign Patents, Washington, DC 20231) Volume(issue)/page/year: #86-05395
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
101 ug/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - mydriasis (pupillary dilation) Sense Organs and Special Senses (Eye) - chromodacryorrhea Blood - changes in spleen
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,653,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
440 ug/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Blood - changes in spleen Skin and Appendages - hair
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 8,560,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
55 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PIXXD2 PCT (Patent Cooperation Treaty) International Application. (U.S. Patent and Trademark Office, Foreign Patents, Washington, DC 20231) Volume(issue)/page/year: #86-05395
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
56 ug/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - dyspnea Musculoskeletal - other changes Skin and Appendages - hair
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 36,323,1976
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
500 ug/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Behavioral - altered sleep time (including change in righting reflex) Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 9,103,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
200 ug/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Behavioral - somnolence (general depressed activity) Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 9,103,1978 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
9100 ng/kg/13W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Endocrine - changes in adrenal weight Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,653,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
375 ug/kg/30D-I
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - changes in calcium Related to Chronic Data - death
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 36,323,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
91 ug/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Endocrine - changes in spleen weight Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 8,589,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
30 ug/kg/30D-I
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain Nutritional and Gross Metabolic - changes in calcium Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 9,103,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
27300 ng/kg/13W-I
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 30,2739,1996
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
30 ug/kg/30D-I
-
TOXIC EFFECTS :
-
Endocrine - changes in thymus weight Nutritional and Gross Metabolic - changes in calcium Related to Chronic Data - death
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 9,103,1978 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1100 ng/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 12,32,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
220 ng/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 12,32,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
520 ng/kg
-
SEX/DURATION :
-
female 17-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 12,716,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
35 ug/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Fertility - mating performance (e.g. # sperm positive females per # females mated; # copulations per # estrus cycles)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 12,203,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3600 ng/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 8,615,1977
|
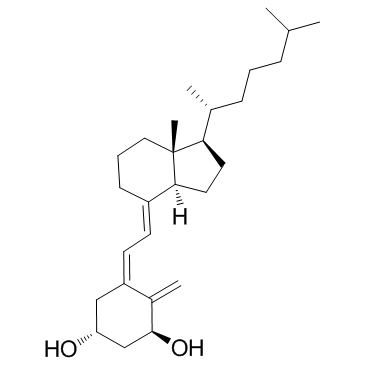


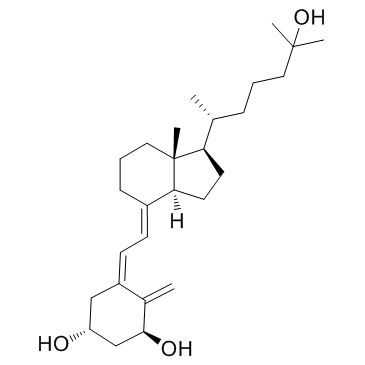 CAS#:32222-06-3
CAS#:32222-06-3 CAS#:112670-85-6
CAS#:112670-85-6
