(S)-(+)-1,2-Propanediol
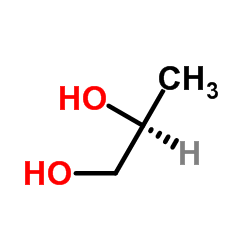
(S)-(+)-1,2-Propanediol structure
|
Common Name | (S)-(+)-1,2-Propanediol | ||
|---|---|---|---|---|
| CAS Number | 4254-15-3 | Molecular Weight | 76.094 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 184.8±8.0 °C at 760 mmHg | |
| Molecular Formula | C3H8O2 | Melting Point | -59ºC | |
| MSDS | Chinese | Flash Point | 107.2±0.0 °C | |
Use of (S)-(+)-1,2-Propanediol(S)-(+)-1,2-Propanediol is an endogenous metabolite. |
| Name | (S)-(+)-1,2-Propanediol |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-(+)-1,2-Propanediol is an endogenous metabolite. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 184.8±8.0 °C at 760 mmHg |
| Melting Point | -59ºC |
| Molecular Formula | C3H8O2 |
| Molecular Weight | 76.094 |
| Flash Point | 107.2±0.0 °C |
| Exact Mass | 76.052429 |
| PSA | 40.46000 |
| LogP | -1.34 |
| Vapour Pressure | 0.2±0.8 mmHg at 25°C |
| Index of Refraction | 1.430 |
| InChIKey | DNIAPMSPPWPWGF-VKHMYHEASA-N |
| SMILES | CC(O)CO |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2905399090 |
|---|---|
| Summary | 2905399090 other diols。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:5.5%。general tariff:30.0% |
|
Percutaneous absorption and distribution of organophosphates (chlorpyrifos and dichlorvos) following dermal exposure and decontamination scenarios using in vitro human skin model.
Toxicol. Lett. 229(1) , 66-72, (2014) To date, there has been little research investigating low-level human exposure to chemicals, and so the aim of this study was to examine the percutaneous penetration of organophosphates (dichlorvos an... |
|
|
Use of a human skin in vitro model to investigate the influence of 'every-day' clothing and skin surface decontamination on the percutaneous penetration of organophosphates.
Toxicol. Lett. 229(1) , 257-64, (2014) Organophosphates (OPs) are widely used in agriculture. Many studies have investigated the capability of personal protective equipment (PPE) to reduce chemical exposure; however, investigations into th... |
|
|
Hydrogen-driven asymmetric reduction of hydroxyacetone to (R)-1,2-propanediol by Ralstonia eutropha transformant expressing alcohol dehydrogenase from Kluyveromyces lactis.
Microb. Cell Fact. 12(1) , 2, (2013) Conversion of industrial processes to more nature-friendly modes is a crucial subject for achieving sustainable development. Utilization of hydrogen-oxidation reactions by hydrogenase as a driving for... |
| Propylene glycol |
| 1,2-Propandiol |
| (2S)-1,2-Propanediol |
| (+)-Propylene glycol |
| UNII:6DC9Q167V3 |
| MFCD00004539 |
| 1,2-Propyleneglycol |
| DL-1,2-PROPANEDIOL |
| (S)-(+)-Propylene glycerol |
| Propane-1,2-diol |
| propanediol |
| (S)-(+)-1,2-DIHYDROXYPROPANE |
| (S)-Propylene Glycol |
| 1,2-(RS)-Propanediol |
| S-1,2-PROPANEDIOL |
| 1,2-propylene glycol |
| (2S)-Propan-1,2-diol |
| (2S)-propane-1,2-diol |
| (±)-1,2-Propanediol |
| (±)-Propylene glycol |
| 1,2-propane-diol |
| PROPYLENE GLYCOL, (S)- |
| 1,2-Propanediol, (2S)- |
| (RS)-1,2-Propanediol |
| (S)-(+)-Propylene oxide |
| (S)-1,2-Propanediol |
| (S)-(+)-Propylene glycol |
| Propan-1,2-diol |
| DL-PROPYLENE GLYCOL |
| 1,2-Propanediol |
| S-(+)-1,2-Propanediol |
| 1,2-Propanediol (8CI,9CI) |
| (S)-propane-1,2-diol |
 CAS#:687-47-8
CAS#:687-47-8 CAS#:85550-19-2
CAS#:85550-19-2 CAS#:116-09-6
CAS#:116-09-6 CAS#:27871-49-4
CAS#:27871-49-4 CAS#:1369852-90-3
CAS#:1369852-90-3 CAS#:17392-83-5
CAS#:17392-83-5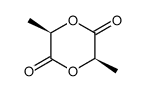 CAS#:13076-17-0
CAS#:13076-17-0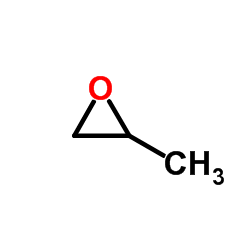 CAS#:75-56-9
CAS#:75-56-9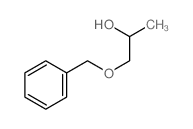 CAS#:13807-91-5
CAS#:13807-91-5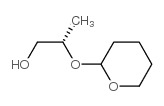 CAS#:76946-21-9
CAS#:76946-21-9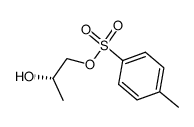 CAS#:32464-98-5
CAS#:32464-98-5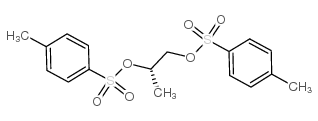 CAS#:60434-71-1
CAS#:60434-71-1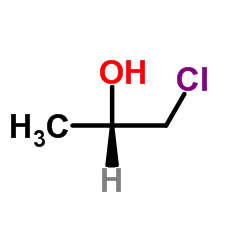 CAS#:37493-16-6
CAS#:37493-16-6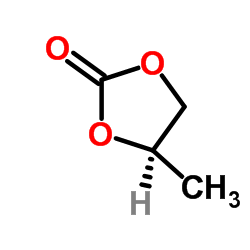 CAS#:51260-39-0
CAS#:51260-39-0 CAS#:33106-64-8
CAS#:33106-64-8 CAS#:15448-47-2
CAS#:15448-47-2 CAS#:16088-62-3
CAS#:16088-62-3![THIENO[3,4-B]-1,4-DIOXIN, 2,3-DIHYDRO-2-METHYL- structure](https://image.chemsrc.com/caspic/029/126235-11-8.png) CAS#:126235-11-8
CAS#:126235-11-8![(2R)-1-[tert-butyl(dimethyl)silyl]oxypropan-2-ol structure](https://image.chemsrc.com/caspic/340/136918-07-5.png) CAS#:136918-07-5
CAS#:136918-07-5 CAS#:16088-60-1
CAS#:16088-60-1
