Luteolin-6-C-glucoside

Luteolin-6-C-glucoside structure
|
Common Name | Luteolin-6-C-glucoside | ||
|---|---|---|---|---|
| CAS Number | 4261-42-1 | Molecular Weight | 448.377 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 856.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C21H20O11 | Melting Point | 245-246ºC | |
| MSDS | Chinese USA | Flash Point | 303.2±27.8 °C | |
Use of Luteolin-6-C-glucosideIsoorientin is a potent inhibitor of COX-2 with an IC50 value of 39 μM. |
| Name | Isoorientin |
|---|---|
| Synonym | More Synonyms |
| Description | Isoorientin is a potent inhibitor of COX-2 with an IC50 value of 39 μM. |
|---|---|
| Related Catalog | |
| Target |
COX-2:39 μM (IC50) |
| In Vitro | Isoorientin is a Selective Inhibitor of Cyclooxygenase-2 (COX-2) from the Tubers of Pueraria tuberosa[1]. PANC-1 and PATU-8988 cells are grown for 24 hours in the presence of Isoorientin (0, 20, 40, 80, and 160 μM), and a CCK8 solution is added. The cell viability decreases significantly at the concentrations of 20, 40, 80, and 160 μM. After the cells are cultured with Isoorientin (0, 20, 40, 80, and 160 μM for PANC-1; 0, 20, 40, 80, 160, and 320 μM for PATU-8988) for 24 hours, the expression of p-AMPK and AMPK is assessed by Western blotting. After the Isoorientin treatment, the p-AMPK expression is increased. Then, in the shRNA group, the concentration of 80 μM is used to detect the effects of Isoorientin. The expression levels of AMPK and p-AMPK are much lower in the shRNA group than in the wild-type PC cells (WT) and the group that is transfected with a negative control lentivirus (NC)[2]. |
| In Vivo | Animals treated with Isoorientin at 10 mg/kg and 20 mg/kg body weight have a statistically significant reduction in paw edema, with a mean peak thickness of 1.19±0.05 mm and 1.08±0.04 mm, respectively. This indicated that Isoorientin significantly attenuates paw edema compared with the control group[3]. |
| Cell Assay | PANC-1 and PATU-8988 cells are plated onto 96-well plates. Each well contain ~5,000 cells and 200 μL of the medium with 10% FBS. When the cells of each well reach 70% confluency, the medium is changed, and FBS-free medium with different concentrations of Isoorientin is added. After 24 hours, the cells are washed with PBS once, the medium containing Isoorientin is discarded, and 100 μL of FBS-free medium with 10 μL of the Cell Counting Kit 8 (CCK8) reagent are added. The cells are incubated for another 1-2 hours at 37°C, and the absorbance of each well is detected using an ELISA reader at 490 nm. Cell viability is expressed as the fold change of absorbance[2]. |
| Animal Admin | Mice[3] In the case of paw edema model the Isoorientin or Celecoxib is given intraperitoneally and Carrageenan is injected into the paw directly one hour later. In air pouch model all the treatments are given along with Carrageenan directly into the pouch cavity. Isoorientin is injected three hours earlier than injection of Carrageenan into the pouch cavity. Isoorientin and Celecoxib are administered into the mice air pouch. The stock solutions of Isoorientin (100 mg/mL) and Celecoxib (100 mg/mL) are prepared in DMSO and further dilutions are made at the time of treatments. Animals are divided, into 5 different groups as follows: control (DMSO treated); Carrageenan (0.5 mL of 1.5% (w/v) Carrageenan in saline) treated; Carrageenan+Celecoxib (20 mg/kg body weight) treated; Carrageenan+Isoorientin (10 mg/Kg body weight) treated; Carrageenan+Isoorientin (20 mg/Kg body weight) treated. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 856.7±65.0 °C at 760 mmHg |
| Melting Point | 245-246ºC |
| Molecular Formula | C21H20O11 |
| Molecular Weight | 448.377 |
| Flash Point | 303.2±27.8 °C |
| Exact Mass | 448.100555 |
| PSA | 201.28000 |
| LogP | 1.58 |
| Appearance of Characters | yellow |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.767 |
| InChIKey | ODBRNZZJSYPIDI-VJXVFPJBSA-N |
| SMILES | O=c1cc(-c2ccc(O)c(O)c2)oc2cc(O)c(C3OC(CO)C(O)C(O)C3O)c(O)c12 |
| Storage condition | 2-8°C |
| Water Solubility | methanol: soluble5mg/mL, clear, colorless to yellow |
|
Nontargeted Analysis Using Ultraperformance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry Uncovers the Effects of Harvest Season on the Metabolites and Taste Quality of Tea (Camellia sinensis L.).
J. Agric. Food Chem. 63 , 9869-78, (2015) The chemical composition and taste quality of tea fluctuate seasonally. However, the compounds responsible for the seasonal variation of metabolic pattern and taste quality are far from clear. This st... |
|
|
Isolation of aspalathin and nothofagin from rooibos (Aspalathus linearis) using high-performance countercurrent chromatography: sample loading and compound stability considerations.
J. Chromatogr. A. 1381 , 29-36, (2015) Aspalathin and nothofagin, the major dihydrochalcones in rooibos (Aspalathus linearis), are valuable bioactive compounds, but their bioactivity has not been fully elucidated. Isolation of these compou... |
|
|
[Protective effect of isoorientin on alcohol-induced hepatic fibrosis in rats].
Zhongguo Zhong Yao Za Zhi 38(21) , 3726-30, (2013) To observe the effect and mechanism of isoorientin from Gypsophila elegans on alcohol-induced hepatic fibrosis in rats.ninety healthy male Wistar rats were randomly divided into six groups: the normal... |
| (1S)-1,5-Anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-6-yl]-D-glucitol |
| D-Glucitol, 1,5-anhydro-1-C-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-1-benzopyran-6-yl]-, (1S)- |
| Lespecapitoside |
| luteolin-6-C-glucoside |
| 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-6-β-D-glucopyranosyl-5,7-dihydroxy- |
| Luteolin6-C-β-D-glucoside,Luteolin6-C-glucoside |
| 2-(3,4-Dihydroxyphényl)-5,7-dihydroxy-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]-4H-chromén-4-one |
| 2-(3,4-Dihydroxyphenyl)-6-b-D-glucopyranosyl-5,7-dihydroxy-4H-1-benzopyran-4-one |
| 2-(3,4-Dihydroxyphenyl)-6-β-D-glucopyranosyl-5,7-dihydroxy-4H-1-benzopyran-4-one |
| Isoorientin |
| 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-4H-chromen-4-one |
| Homoorientin |
| 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-4H-chromen-4-on |
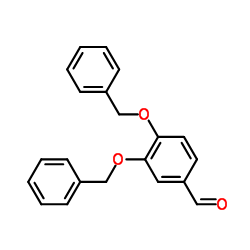 CAS#:5447-02-9
CAS#:5447-02-9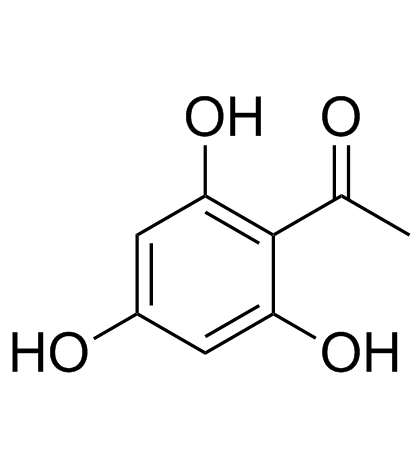 CAS#:480-66-0
CAS#:480-66-0![1-[2-hydroxy-4,6-bis(methoxymethoxy)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/448/65490-09-7.png) CAS#:65490-09-7
CAS#:65490-09-7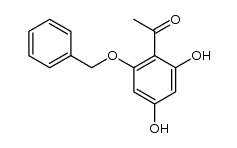 CAS#:39548-86-2
CAS#:39548-86-2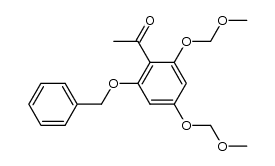 CAS#:115623-29-5
CAS#:115623-29-5
