1-Iodononane
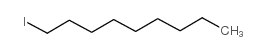
1-Iodononane structure
|
Common Name | 1-Iodononane | ||
|---|---|---|---|---|
| CAS Number | 4282-42-2 | Molecular Weight | 254.15200 | |
| Density | 1.288 | Boiling Point | 107-108 °C (8 mmHg) | |
| Molecular Formula | C9H19I | Melting Point | -20ºC | |
| MSDS | Chinese USA | Flash Point | 85 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 1-Iodononane |
|---|---|
| Synonym | More Synonyms |
| Density | 1.288 |
|---|---|
| Boiling Point | 107-108 °C (8 mmHg) |
| Melting Point | -20ºC |
| Molecular Formula | C9H19I |
| Molecular Weight | 254.15200 |
| Flash Point | 85 °C |
| Exact Mass | 254.05300 |
| LogP | 4.17200 |
| Index of Refraction | 1.486-1.488 |
| InChIKey | OGSJMFCWOUHXHN-UHFFFAOYSA-N |
| SMILES | CCCCCCCCCI |
| Stability | Stable. Incompatible with strong oxidizing agents, strong bases. May discolour upon exposure to light. |
| Water Solubility | insoluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S37/39-S26-S36/37/39 |
| HS Code | 2903399090 |
|
~43% 
1-Iodononane CAS#:4282-42-2 |
| Literature: Olah, George A.; Husain, Altaf; Singh, Brij P.; Mehrotra, Ashok K. Journal of Organic Chemistry, 1983 , vol. 48, # 21 p. 3667 - 3672 |
|
~% 
1-Iodononane CAS#:4282-42-2 |
| Literature: Feshchenko,N.G.; Kirsanov,A.V. J. Gen. Chem. USSR (Engl. Transl.), 1966 , vol. 36, # 1 p. 157 - 159,162 - 164 |
|
~42% 
1-Iodononane CAS#:4282-42-2
Detail
|
| Literature: Olah, George A.; Husain, Altaf; Gupta, B. G. Balaram; Narang, Subhash C. Angewandte Chemie, 1981 , vol. 93, # 8 p. 705 - 706 |
|
~% 
1-Iodononane CAS#:4282-42-2 |
| Literature: Shono; Chuankamnerdkarn; Maekawa; Ishifune; Kashimura Synthesis, 1994 , # 9 p. 895 - 897 |
|
~% 
1-Iodononane CAS#:4282-42-2 |
| Literature: Shono; Chuankamnerdkarn; Maekawa; Ishifune; Kashimura Synthesis, 1994 , # 9 p. 895 - 897 |
|
~% 
1-Iodononane CAS#:4282-42-2 |
| Literature: Krafft Chemische Berichte, 1886 , vol. 19, p. 2221 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2903399090 |
|---|---|
| Summary | 2903399090. brominated,fluorinated or iodinated derivatives of acyclic hydrocarbons. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Synthesis of phalluside-1 and Sch II using 1,2-metallate rearrangements.
Org. Biomol. Chem. 8(5) , 1188-93, (2010) (4E,8E,10E)-9-Methyl-4,8,10-sphingatrienine, a core component of marine sphingolipids, was synthesised for the first time using a copper(I)-mediated 1,2-metallate rearrangement of a lithiated glycal a... |
|
|
Sulfone-mediated synthesis of polysubstituted pyridines. Craig D and Henry GD.
Tetrahedron Lett. 46(15) , 2559-2562, (2005)
|
|
|
Ultrasound mediated synthesis of a few naturally occurring compounds. Singh J, et al.
Indian J. Chem. B 40(5) , 386-90, (2005)
|
| 1-Jodnonan |
| 1-n-nonyl iodide |
| EINECS 224-286-3 |
| n-nonyl iodide |
| MFCD00001107 |
| 1-Iodononane |
| Nonyl Iodide |
| 1-iodo-n-nonane |
| Nonane,1-iodo |
| 1-iodo-nonan |



 CAS#:45206-91-5
CAS#:45206-91-5 CAS#:593-08-8
CAS#:593-08-8 CAS#:593-45-3
CAS#:593-45-3 CAS#:929-98-6
CAS#:929-98-6 CAS#:50262-46-9
CAS#:50262-46-9 CAS#:872-05-9
CAS#:872-05-9 CAS#:2456-27-1
CAS#:2456-27-1 CAS#:24323-25-9
CAS#:24323-25-9 CAS#:111-84-2
CAS#:111-84-2 CAS#:152634-09-8
CAS#:152634-09-8