Glycyrrhetinic acid
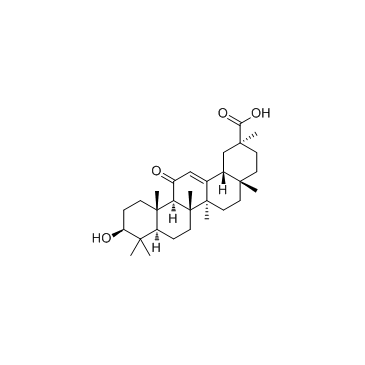
Glycyrrhetinic acid structure
|
Common Name | Glycyrrhetinic acid | ||
|---|---|---|---|---|
| CAS Number | 471-53-4 | Molecular Weight | 470.684 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 588.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C30H46O4 | Melting Point | 292 - 295ºC | |
| MSDS | N/A | Flash Point | 323.7±26.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Glycyrrhetinic acid18β-Glycyrrhetinic acid is the major bioactive component of Glycyrrhizae Radix and possesses anti-ulcerative, anti-inflammatory and antiproliferative properties. |
| Name | Enoxolone |
|---|---|
| Synonym | More Synonyms |
| Description | 18β-Glycyrrhetinic acid is the major bioactive component of Glycyrrhizae Radix and possesses anti-ulcerative, anti-inflammatory and antiproliferative properties. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | 18β-Glycyrrhetinic acid is the major bioactive component of Glycyrrhizae Radix and possesses anti-ulcerative, anti-inflammatory and antiproliferative properties. MTS assay demonstrates that 24 h treatment of 18β-Glycyrrhetinic acid suppresses cell proliferation in both cell lines in a dose-dependent manner. 18β-Glycyrrhetinic acid at 160 μM significantly decreases the percentage of viable cells to around 40.5±10.5% in A549 and 38.3±4.6% in NCI-H460 (p<0.01 respectively). When the cells are treated with 320 μM 18β-Glycyrrhetinic acid, a greater inhibitory effects on cell proliferation is shown, as the percentage of viable cells is below 30% compare with untreated controls (p<0.001). Treatment with 18β-Glycyrrhetinic acid at 160 μM and 320 μM decreases the levels of full-length PARP and increases the levels of cleaved-PARP[1]. |
| In Vivo | Rats in 18β-Glycyrrhetinic acid+Triptolide (TP) group which receive low-dose 18β-Glycyrrhetinic acid (50 mg/kg) have significant reductions in the three serum parameters when compare with TP rats. Rats in 18β-Glycyrrhetinic acid+TP group which receive the high-dose 18β-Glycyrrhetinic acid (100 mg/kg) have slightly lowered the levels of three liver enzymes, the reductions do not reach statistical significance compare with TP group. Contrastingly, preadministration of low-dose 18β-Glycyrrhetinic acid protects animals from TP-induced hepatic lesions. On the contrary, low-dose 18β-Glycyrrhetinic acid (50 mg/kg) markedly suppresses the release of the four cytokines above[3]. |
| Cell Assay | Primary microglia cultures are used in this study. For treatment assay, microglia are incubated with complete DMEM and stimulated with or without 100 ng/mL IFN-γ in the presence or absence of 18β-Glycyrrhetinic acid (25 μM and 50 μM) at 37°C in a humidified incubator with 5% CO2. For cell migration assay, the isolated primary microglia that seeded in complete DMEM medium are stimulated with or without IFN-γ (100 ng/mL), and treated with different doses of 18β-Glycyrrhetinic acid, 24 h later, the microglia culture supernatants are collected and added to the lower chambers of Transwell inserts[2]. |
| Animal Admin | Healthy Wistar rats (male, 200±20 g) are used and divided into five groups with 10 individuals for each group randomly. Animals in normal control (NC) group receive distilled water for 6 days and 0.5% CMC-Na for the last 3 days. Rats in Triptolide model group (TP), 18β-Glycyrrhetinic acid low-dose group (GAL+TP), and 18β-Glycyrrhetinic acid high-dose group (GAH+TP) receive distilled water, 18β-Glycyrrhetinic acid (50 mg/kg, p.o., dissolved in distilled water), or 18β-Glycyrrhetinic acid (100 mg/kg, p.o., dissolved in distilled water) for consecutive 6 days, respectively, and liver injury is induced by TP (2.4 mg/kg, p.o., suspended in 0.5% CMC-Na) for the last 3 days. Animals in the above three groups receive TP 6 hours after distilled water or 18β-Glycyrrhetinic acid treatment on the last 3 days[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 588.3±50.0 °C at 760 mmHg |
| Melting Point | 292 - 295ºC |
| Molecular Formula | C30H46O4 |
| Molecular Weight | 470.684 |
| Flash Point | 323.7±26.6 °C |
| Exact Mass | 470.339600 |
| PSA | 74.60000 |
| LogP | 6.57 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.563 |
| InChIKey | MPDGHEJMBKOTSU-YKLVYJNSSA-N |
| SMILES | CC1(C(=O)O)CCC2(C)CCC3(C)C(=CC(=O)C4C5(C)CCC(O)C(C)(C)C5CCC43C)C2C1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P301 + P312 + P330-P305 + P351 + P338 |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22;R36 |
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | RK0180000 |
| HS Code | 2938909030 |
| Precursor 8 | |
|---|---|
| DownStream 6 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Hepatocellular carcinoma dually-targeted nanoparticles for reduction triggered intracellular delivery of doxorubicin.
Int. J. Pharm. 478(2) , 553-68, (2015) Hepatocellular carcinoma (HCC) dual targeted stimuli responsive nanoparticles (NPs) for intracellular delivery of doxorubicin (DOX) were developed based on a reduction cleavable hyaluronic acid-glycyr... |
|
|
Two-step inhibitory effect of kanzo on oxytocin-induced and prostaglandin F2α-induced uterine myometrial contractions.
J. Nat. Med. 68(3) , 550-60, (2014) We previously reported that shakuyaku-kanzo-to, a kampo medicine consisting of shakuyaku and kanzo, has an inhibitory effect on myometrial contractions in pregnant women. In this study, we evaluated t... |
|
|
Prevention of cisplatin-induced ototoxicity by the inhibition of gap junctional intercellular communication in auditory cells. Kim YJ, Kim J, Tian C, et al.
Cell. Mol. Life Sci. 71(19) , 3859-71, (2014)
|
| MFCD00003706 |
| (3β)-3-Hydroxy-11-oxoolean-12-en-30-oic acid |
| Glycyrrhetinic acid |
| (2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-Hydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-13-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydro-2-picenecarboxylic acid |
| 18-beta-Glycyrrhetinic acid |
| Arthrodont |
| EINECS 207-444-6 |
| 18-β-Glycyrrhetinic Acid |
| glycyrrhetin |
| GM 1658 |
| Uralenic acid |
| 3β-Hydroxy-11-oxoolean-12-en-30-oic acid |
| 18b-Glycyrrhetic acid |
| Biosone |
| Olean-12-en-30-oic acid, 3β-hydroxy-11-oxo- |
| 18β-Glycyrrhetinic acid |
| STX 352 |
| Glycyrrhetic Acid |
| PO 12 |
| Olean-12-en-30-oic acid, 3-hydroxy-11-oxo-, (3β)- |
| 3b-Hydroxy-11-oxoolean-12-en-30-oic Acid |
| 18b-Glycyrrhetinic Acid |
| Glycyrrhetinate |
| (3b,20b)-3-Hydroxy-11-oxoolean-12-en-29-oic Acid |
| ENOLOXONE |
| enoxolone |
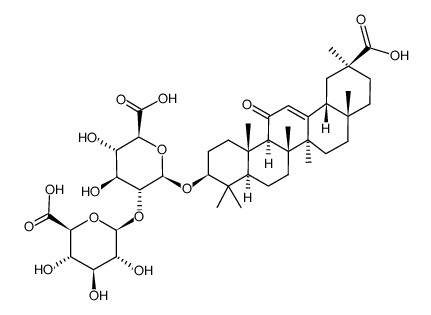 CAS#:83896-44-0
CAS#:83896-44-0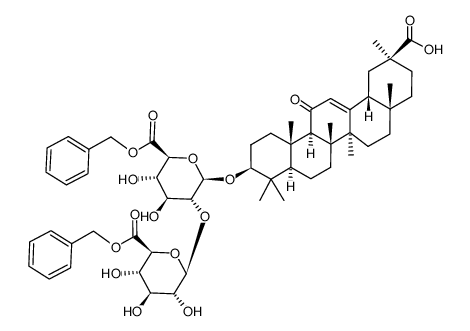 CAS#:87918-97-6
CAS#:87918-97-6 CAS#:1405-86-3
CAS#:1405-86-3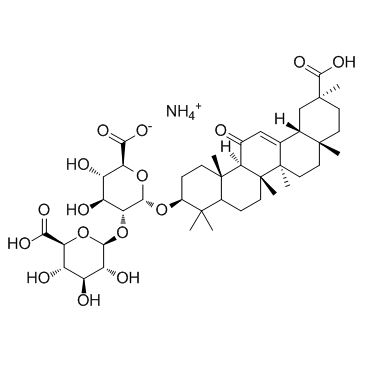 CAS#:53956-04-0
CAS#:53956-04-0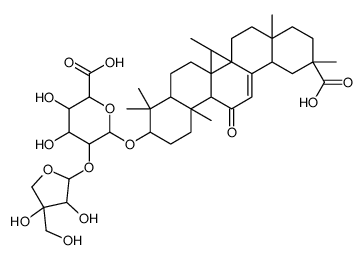 CAS#:121709-66-8
CAS#:121709-66-8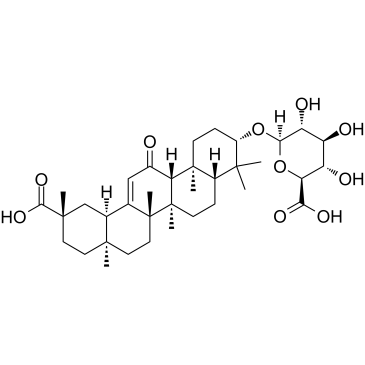 CAS#:34096-83-8
CAS#:34096-83-8 CAS#:121687-83-0
CAS#:121687-83-0 CAS#:67-56-1
CAS#:67-56-1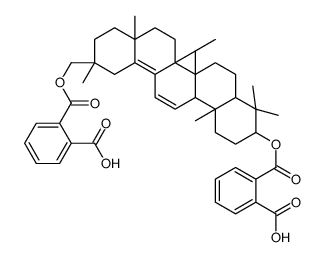 CAS#:102416-29-5
CAS#:102416-29-5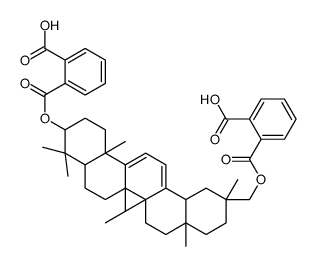 CAS#:102416-28-4
CAS#:102416-28-4 CAS#:13832-70-7
CAS#:13832-70-7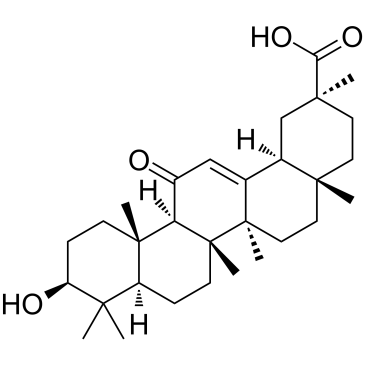 CAS#:1449-05-4
CAS#:1449-05-4 CAS#:486-34-0
CAS#:486-34-0
