Chrysin
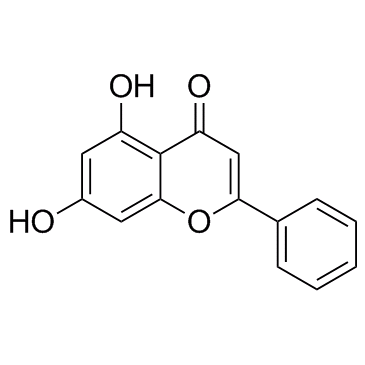
Chrysin structure
|
Common Name | Chrysin | ||
|---|---|---|---|---|
| CAS Number | 480-40-0 | Molecular Weight | 254.238 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 491.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C15H10O4 | Melting Point | 284-286 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 192.5±22.2 °C | |
Use of ChrysinChrysin is one of the most well known estrogen blockers. |
| Name | chrysin |
|---|---|
| Synonym | More Synonyms |
| Description | Chrysin is one of the most well known estrogen blockers. |
|---|---|
| Related Catalog | |
| Target |
estrogen |
| In Vitro | Chrysin is mainly found in passion flowers, honey, and propolis acts as a potential therapeutic and preventive agent to inhibit proliferation and invasion of various human cancer cells. Although Chrysin has anti-carcinogenic effects in several cancers, little is known about its functional roles in ovarian cancer which shows poor prognosis and chemoresistance to traditional therapeutic agents. Chrysin inhibits ovarian cancer cell proliferation and induced cell death by increasing reactive oxygen species (ROS) production and cytoplasmic Ca2+ levels as well as inducing loss of mitochondrial membrane potential (MMP). Chrysin activates MAPK and PI3K/AKT pathways in ES2 and OV90 cells in concentration-response experiments. Chrysin suppresses tumor growth byregulating canonical Wnt and nuclear factor NF-κB signaling cascades cancer cells. Chrysin stimulates the phosphorylation of AKT and P70S6K proteins in both ES2 and OV90 cells compared tothe untreated control cells. In addition, Chrysin activates the phospho-ERK1/2, p38,and JNK proteins as members of the MAPK pathway in the ovarian cancer cells[1]. |
| Cell Assay | The proliferation assays are conducted using a cell proliferation enzyme-linked immunosorbent assay (ELISA) 5-bromo-2'-deoxyuridine (BrdU) kit. Briefly, ES2 and OV90 cells are seeded in a 96-well plate, and then treated with Chrysin (0, 5, 10, 20, 50, and 100 µM) with or without inhibitors (20 μM LY294002, PI3K/AKT; 10 μM U0126, ERK1/2; 10 μM SP600125, JNK; and 20 μM SB203580, p38) in a final volume of 100 μL/well. Aftera48-h incubation, 10 μM BrdU is added to the cell culture, followed by an additional 2-h incubation at 37°C. After labeling the cells with BrdU, they are fixed and then incubated with the anti-BrdU-peroxidase (POD) working solution for 90 min. The anti-BrdU-POD binds to the BrdU incorporated into newly synthesized cellular DNA and these immune complexes are detected by analyzing their reaction with the 3,3',5,5'-tetramethylbenzidine (TMB) substrate. The absorbance values of the reaction product are measured at 370 and 492 nm using an ELISA reader[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 491.9±45.0 °C at 760 mmHg |
| Melting Point | 284-286 °C(lit.) |
| Molecular Formula | C15H10O4 |
| Molecular Weight | 254.238 |
| Flash Point | 192.5±22.2 °C |
| Exact Mass | 254.057907 |
| PSA | 70.67000 |
| LogP | 2.88 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.699 |
| Storage condition | 0-6°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LK8329050 |
| HS Code | 2914400090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914400090 |
|---|---|
| Summary | 2914400090 other ketone-alcohols and ketone-aldehydes。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems.
Talanta 140 , 134-42, (2015) An important trend in the extraction of chemical compounds is the application of new environmentally friendly, food grade solvents. Ethyl lactate (ethyl 2-hydroxypropanoate), produced by fermentation ... |
|
|
Ultra high performance liquid chromatography with mass spectrometry method for the simultaneous determination of phenolic constituents in honey from various floral sources using multiwalled carbon nanotubes as extraction sorbents.
J. Sep. Sci. 38 , 2597-606, (2015) An ultra high performance liquid chromatography with mass spectrometry method has been developed for the simultaneous separation, identification and determination of 22 phenolic constituents in honey ... |
|
|
Combined experimental and in silico approaches for exploring antiperoxidative potential of structurally diverse classes of antioxidants on docetaxel-induced lipid peroxidation using 4-HNE as the model marker.
Bioorg. Chem. 56 , 1-7, (2014) The objective of the present work was tantamount to explain the antiperoxidative potential and structural requirements of twenty-eight structurally diverse classes of antioxidants on docetaxel-induced... |
| 5,7-Dihydroxyflavone |
| Chrysin |
| 5,7-dihydroxy-2-phenylchromen-4-one |
| EINECS 207-549-7 |
| 5,7-Dihydroxy-2-phenyl-4H-chromen-4-one |
| MFCD00006834 |
| Chrysidenon 1438 |
| Chrysinic acid |
| 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-phenyl- |
 CAS#:21392-57-4
CAS#:21392-57-4 CAS#:480-66-0
CAS#:480-66-0 CAS#:98-88-4
CAS#:98-88-4 CAS#:6665-78-7
CAS#:6665-78-7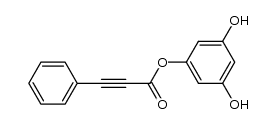 CAS#:396103-20-1
CAS#:396103-20-1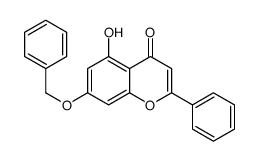 CAS#:110506-85-9
CAS#:110506-85-9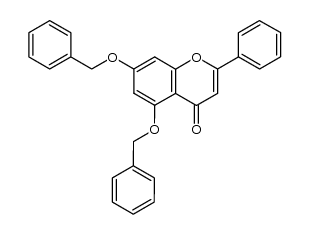 CAS#:137790-18-2
CAS#:137790-18-2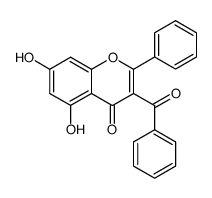 CAS#:856406-49-0
CAS#:856406-49-0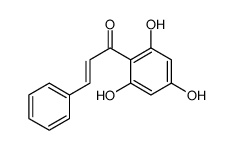 CAS#:82451-30-7
CAS#:82451-30-7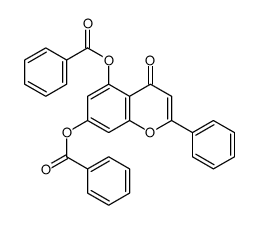 CAS#:110865-06-0
CAS#:110865-06-0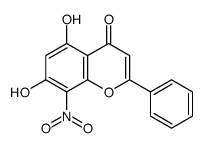 CAS#:105173-18-0
CAS#:105173-18-0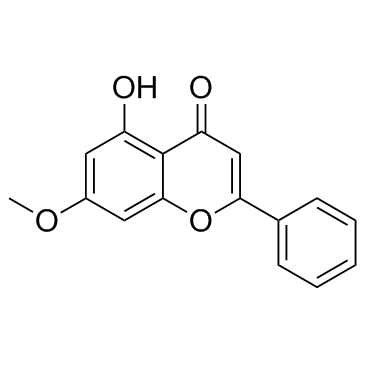 CAS#:520-28-5
CAS#:520-28-5 CAS#:1707-75-1
CAS#:1707-75-1 CAS#:2255-62-1
CAS#:2255-62-1 CAS#:2520-36-7
CAS#:2520-36-7 CAS#:632-85-9
CAS#:632-85-9 CAS#:6674-40-4
CAS#:6674-40-4
