AZD2858
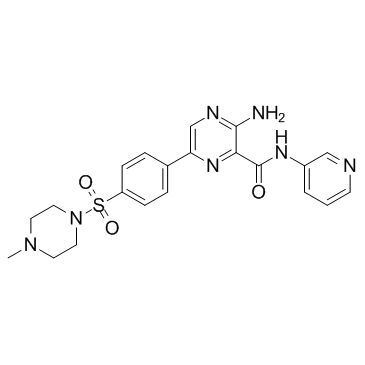
AZD2858 structure
|
Common Name | AZD2858 | ||
|---|---|---|---|---|
| CAS Number | 486424-20-8 | Molecular Weight | 453.517 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C21H23N7O3S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of AZD2858AZD2858 is a potent, orally active GSK-3 inhibitor, with IC50s of 0.9 and 5 nM for GSK-3α and GSK-3β, respectively, used in the research of fracture healing. |
| Name | 3-Amino-6-{4-[(4-methyl-1-piperazinyl)sulfonyl]phenyl}-N-(3-pyrid inyl)-2-pyrazinecarboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | AZD2858 is a potent, orally active GSK-3 inhibitor, with IC50s of 0.9 and 5 nM for GSK-3α and GSK-3β, respectively, used in the research of fracture healing. |
|---|---|
| Related Catalog | |
| Target |
GSK-3α:0.9 nM (IC50) GSK-3β:5 nM (IC50) CDK5/p25:356 nM (IC50) Haspin:366 nM (IC50) CDK5/p35:387 nM (IC50) DYRK2:491 nM (IC50) CDK2/cyclin A:810 nM (IC50) CDK1/cyclin B:1246 nM (IC50) PIM3:1269 nM (IC50) TLK2:1381 nM (IC50) PKD2:2462 nM (IC50) CDK2/cyclin E:3310 nM (IC50) Aurora-A:4966 nM (IC50) |
| In Vitro | AZD2858 (1 μM) increases β-catenin levels after a short period of time in human osteoblast cells. AZD2858 inhibits GSK-3β dependent phosphorylation with an IC50 of 68 nM. AZD2858 (10 nM) has no effect on β-catenin levels[1]. AZD2858 increases TAZ expression and osterix expression both by 1.4-fold, with EC50 of 440 nM and 1.2 μM, respectively, in hADSC. AZD2858 also induces a marked increase in osteogenic mineralisation in hADSC[3]. AZD2858 (AR28) demonstrates from 70- to greater than 6000-fold selectivity over a panel of other kinases and an IC50 of 5 nM. AR28 inhibits GSK-3 in murine cells and indicates activation of the canonical Wnt/β-catenin signaling cascade. AR28 (50, 10, and 1 nM) enhances the clonogenic ability of mesenchymal progenitors with osteogenic and adipogenic potential. AR28 (50 µM) also enhances the differentiation ability of mesenchymal progenitors to the osteogenic but not adipogenic lineage in vitro[4]. |
| In Vivo | AZD2858 (20 mg/kg) causes a dose-dependent increase in trabecular bone mass compared to control after a two-week treatment with a maximum effect[1]. AZD2858 exhibits a substantial effect on fracture healing. AZD2858 (20 mg/kg) causes an increase in cortical BMC of 9%, cortical area of 10%, and cortical thickness of 11% at 3 weeks in the non-operated right femur of rats[2]. AZD2858 (30 μmol/kg/day) alters the biomarkers of bone turnover with statistically significant increases in P1NP and decreases in TRAcP-5b seen from 3 days of treatment and onwards. AZD2858 demonstrates significant changes in serum bone turnover markers (P1NP and TRAcP-5b) and femur bone formation after only 7 days of daily dosing[3]. AZD2858 (AR28, 30 mg/kg, s.c.) stimulates an increase in an initial wave of mesenchymal progenitors with osteogenic and adipogenic potential and drives their differentiation to the osteogenic lineage in BALB/c mice. AR28 (30 mg/kg, s.c.) enhances the proliferation of committed hematopoietic progenitors and their differentiation to the osteoclast lineage but does not prevent an overall increase in bone mass[4]. |
| Kinase Assay | The potency of compounds at GSK-3β and cyclin-dependent protein kinase 2 (CDK2, kinase with closest homology to GSK-3β) is assessed using Z-LYTE™ Kinase assay kit in the presence of 7 and 80 μM ATP respectively. A ratiometric method is used to calculate the ratio of donor emission (445 nm) to acceptor emission (520 nm) after excitation of the donor fluorophore at 400 nm to quantitate the reaction progress. Kinase selectivity with AR79, AZD2858 and AZ13282107 are determined using the KinaseProfiler Service or University of Dundee Kinase. Over 80 different kinases are assessed at a single concentration of 1 or 10 μM of AR79, AZD2858 and AZ13282107. Concentration-inhibition 10-point curves to compounds that show activity are constructed to determine pIC50 estimations. Also, in some kinase assays these pIC50 estimations are converted to binding affinity values (pKi) using the Cheng-Prussoff equation to correct for the concentration of ATP used[3]. |
| Animal Admin | Each rat is dosed orally with vehicle or AZD2858 using a plastic gavage tube. The dose volume is 10 mL/kg. The vehicle consists of deionized water adjusted to pH 3.5±0.1. Formulations are adjusted to pH 3.5±0.1. The doses are 0, 0.2, 2 or 20 mg/kg respectively and administered either twice daily (TD), once daily (OD), every other day (O/2D), or every fourth day (O/4D) for 14 days. In total, the protocol results in 13 groups with 8 animals in each group (104 animals). At 7 days after the start of the study, and again 2 days prior to the scheduled terminal necropsy, each animal is injected subcutaneously with a bicarbonate buffered calcein solution (8 mg/kg, 1 mL/kg)[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C21H23N7O3S |
| Molecular Weight | 453.517 |
| Exact Mass | 453.158295 |
| PSA | 146.28000 |
| LogP | 2.97 |
| Index of Refraction | 1.663 |
| InChIKey | FHCSBLWRGCOVPT-UHFFFAOYSA-N |
| SMILES | CN1CCN(S(=O)(=O)c2ccc(-c3cnc(N)c(C(=O)Nc4cccnc4)n3)cc2)CC1 |
| Storage condition | -20°C |
| Hazard Codes | T+ |
|---|---|
| RIDADR | 2811.0 |
| 3-Amino-6-{4-[(4-methyl-1-piperazinyl)sulfonyl]phenyl}-N-(3-pyridinyl)-2-pyrazinecarboxamide |
| 2-Pyrazinecarboxamide, 3-amino-6-[4-[(4-methyl-1-piperazinyl)sulfonyl]phenyl]-N-3-pyridinyl- |
| AZD2858 |